88
VOLUME 11 NUMBER 2 • JUNE 2014
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
2 diabetes presented with a more lethal phenotype and a higher
mortality rate. Also diabetic complications were detected more
frequently.
10
Pathophysiology/aetiology
Long-term hyperglycaemia, both in type 1 and type 2 diabetes, leads
to microvascular and macrovascular complications.
11
Microvascular
damage affects particularly the retina, kidneys, and both the
autonomic and peripheral nervous system, while the heart, brain
and lower limbs are affected by both micro- and macrovascular
disorders.
11
Oxidative stress
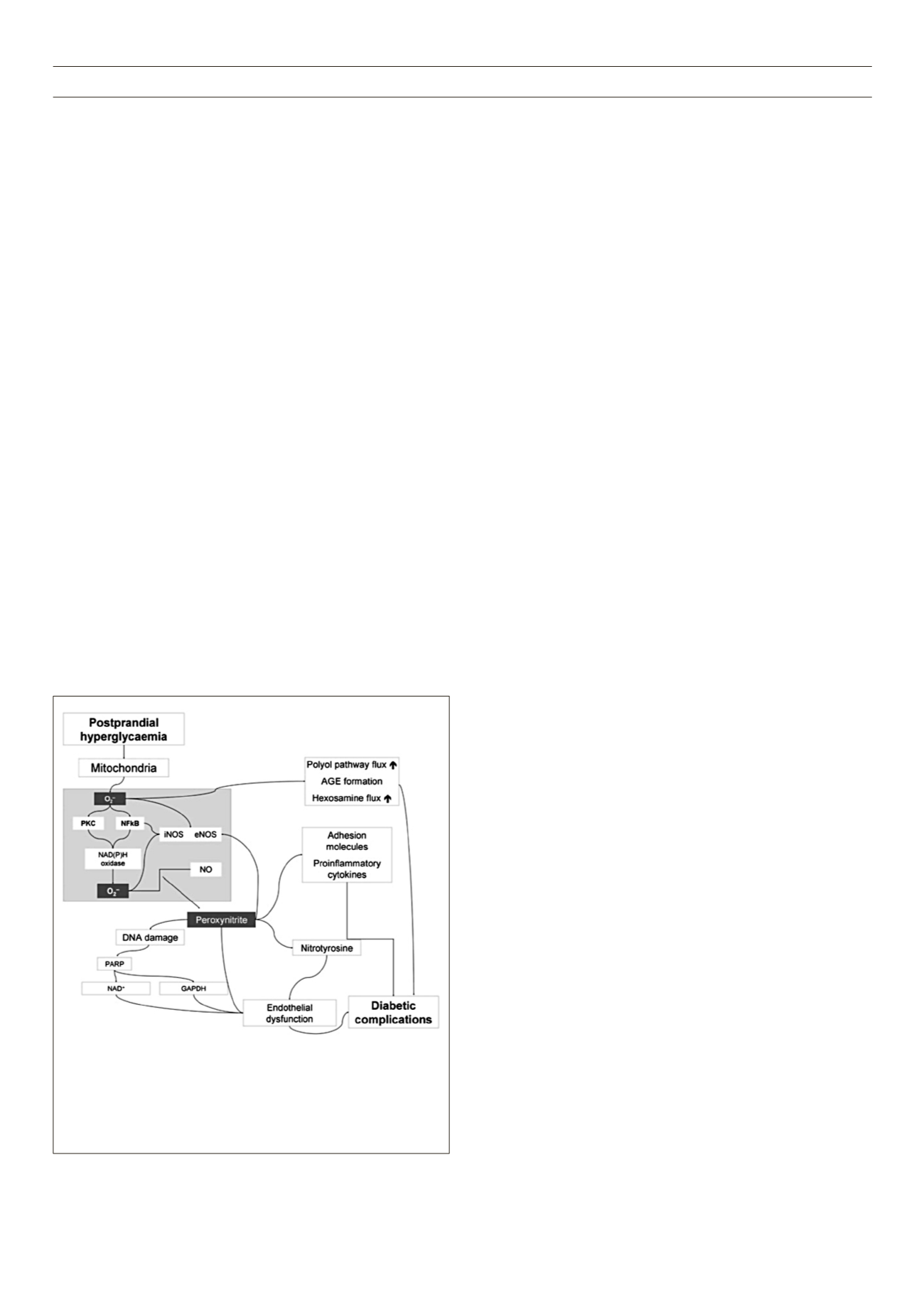
Hyperglycaemia-induced overproduction of superoxide by the
mitochondrial electron transport chain is supposed to be the key
element in the activation of all other pathways involved in the
pathogenesis of diabetic complications (Fig. 1).
12,13
These include
an increase in polyol pathway flux and advanced glycation end-
product formation, an activation of protein kinase C, and an
increase in hexosamine pathway flux. Superoxide overproduction
is accompanied by increased nitric oxide generation, due to an
endothelial nitric oxide synthase (NOS) and inducible NOS uncoupled
state. Therefore the formation of the strong oxidant peroxynitrite
is favoured, which in turn damages the deoxyribonucleic acid
(DNA).
12,13
Due to this DNA damage, a rapid activation of poly[adenosine
diphosphate (ADP)-ribose] polymerase occurs, in turn depleting
the intracellular concentration of its substrate nicotinamide
adenine dinucleotide (NAD+), and slowing the rate of glycolysis,
electron transport, and adenosintriphosphate (ATP) formation. In
addition, the ADP-ribosylation of the glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) is stimulated. These processes result in
acute endothelial dysfunction, which contributes to the genesis of
diabetic complications.
12,13
Inflammation
An increase in inflammatory cytokines is also proposed to
contribute to plaque instability in patients with diabetes.
14
Several
inflammatory markers including C-reactive protein, interleukin
(IL)-6, IL-8, tumour necrosis factor (TNF)-
α
, and endothelin-1 are
increased during hypoglycaemia. The accumulation of inflammatory
cytokines is assumed to cause endothelial injury and abnormalities
in coagulation, resulting in increased risk for CV events.
14
Hypercoagulability
The coagulation system is altered due to changes in clotting factor
levels and/or activity. Plasma levels of procoagulant factors are
increasedwhile fibrinolytic capacity is decreased.
15
Hyperinsulinaemia
results in increased hepatic synthesis of prothrombotic factors
such as fibrinogen and plasminogen activator inhibitor (PAI)-1,
thereby creating a thrombotic milieu. Furthermore, diabetes causes
quantitative modifications in clotting factors, including glycation
and oxidation which also increase thrombosis risk.
15
Autonomic neuropathy
Cardiac autonomic neuropathy (CAN) detected by standard tests
is a common complication of type 1 diabetes. CAN prevalence is
around 20% and increases with age and diabetes duration with
about a 2% annual increase.
16
Poor glycaemic control is a strong
risk factor for CAN as supported by the EURODIAB study.
17
In the
Diabetes Control and Complications Trial (DCCT), intensive insulin
treatment reduced the incidence of CAN by 53% compared
to conventional therapy.
18
In the Epidemiology of Diabetes
Interventions and Complications (EDIC) study, at the 13th to 14th
year after DCCT close-out, the prevalence and incidence of CAN
remained significantly lower in the former intensive than in the
former conventional group.
19
Several studies showed the predictive value of CAN on
mortality,
16
and that CAN is an independent predictor of mortality.
CAN was reported to be a predictor of CV morbidity and mortality
in type 1 diabetes.
20
Various CV disorders associated with CAN and
resulting from vagal impairment and sympathetic predominance
were shown mostly in type 2 diabetes and may account for the
poor prognosis related to CAN.
16
Such disorders have been far less
studied in patients with type 1 diabetes.
In a study on patients with type 1 and type 2 diabetes, the
prevalence of hypertension was shown to increase with CAN
severity (from 3.6% in the patients without CAN to 36.4% in those
with severe CAN), and CAN was an independent risk factor for
hypertension.
21
This association suggests that vagosympathetic
imbalance with a relative sympathetic overdrive may be involved
in hypertension. In the Pittsburgh EDC study, CAN was associated
with increased arterial stiffness 18 years later.
22
There is also strong
evidence, based on studies in patients with type 1 or type 2
diabetes, that QT-interval prolongation is an independent predictor
of mortality for all-cause and cardiovascular deaths.
16
The balance of the activity of the autonomic nervous system is
considered to play a key role in the performance of the diabetic
heart.
23
Advanced single-photon emission computed tomography
(SPECT) and positron emission tomography (PET) allow one to
directly and sensitively assess cardiac sympathetic innervation,
24-29
Figure 1.
Pathogenesis of diabetic complications: hyperglycaemia-induced
overproduction of superoxide by the mitochondrial electron transport chain
is proposed to be the key element. By activation of different pathways, the
formation of the strong oxidant peroxynitrite is favoured, which in turn
damages the DNA. Through several intermediate steps, acute endothelial
dysfunctioncontributing to the genesis of diabetic complications, is triggered.
13


