
168
VOLUME 11 NUMBER 4 • NOVEMBER 2014
LEARNING FROM PRACTICE
SA JOURNAL OF DIABETES & VASCULAR DISEASE
(53–110),
albumin:creatinineratio (ACR) 1.5 ± 2.4 mg/mmol (0.3–
7.5), cholesterol 4.4 ± 0.8 mmol/l (3.2–5.5), C-peptide was negative
{< 94 pmol/l [analysed by the Mercodia C-peptide enzyme-linked
immunosorbent assay (ELISA) assay]} in six patients and low in two
(281, 131 pmol/l), retinopathy status (none = 1, background = 2,
pre-proliferative and above = 5), foot risk (low risk = 5, intermediate
risk = 3) and only one patient had macrovascular complications.
On an intention-to-treat basis at three, six and 12 months,
weight loss was 6.8 ± 4.1 kg, 10.0 ± 5.6 kg and 9.0 ± 8.5 kg (range
–21 to +6.8 kg) (p = 0.026). Percentage weight loss at year end
was 8 ± 6% (range +4 to –16%). Daily insulin dose fell by 52 ± 69
units, 50 ± 69 units and 43 ± 60 units (median 16, range –168 to
+6 units) (
p
= 0.107, ns). Insulin dosage in units/kg was 1.0 ± 0.9,
0.7 ± 0.4, 0.7 ± 0.4 and 0.7 ± 0.6 (
p
= 0.136, ns). HbA
1c
changes
were not significant (
p
= 0.962, ns).
Two patients were unable to tolerate liraglutide and withdrew
at six months. They are indicated in Fig. 1. In one there was no
response in any parameter (HbA
1c
, weight or insulin dose), also
mandating withdrawal. In the other, weight and insulin dosage
rose following cessation of GLP-1 therapy. Excluding these two
cases (
n
= 6), insulin dose reduction over one year was significant
(
p
= 0.044) at 12 months (–44 ± 66 units per day) but with no
significant difference when assessed by units/kg (
p
= 0.158, ns).
Percentage weight loss at year end was 11 ± 3% (range –7 to
–16%,
p
= 0.003).
Alternatively, analysis to the six-month time point (
n
= 8) showed
significant falls in weight (
p
= 0.021) and a significant reduction in
insulin either by total daily dose (
p
= 0.045) or in daily units/kg (
p
=
0.044) while HbA
1c
remained static.
There were no significant hypoglycaemic events nor any episodes
of acute metabolic destabilisation.
Discussion
Under this tightly observed protocol, in motivated patients with
type 1 diabetes, under close clinical supervision (and by whatever
mechanisms of action
14-17
), significant weight reduction occurred
without metabolic destabilisation. Clinically and statistically signifi-
cant reductions in insulin dosages were achieved, which appeared
to be a consequence of the weight loss possibly indicative of an
improvement in insulin resistance as determined by the crude
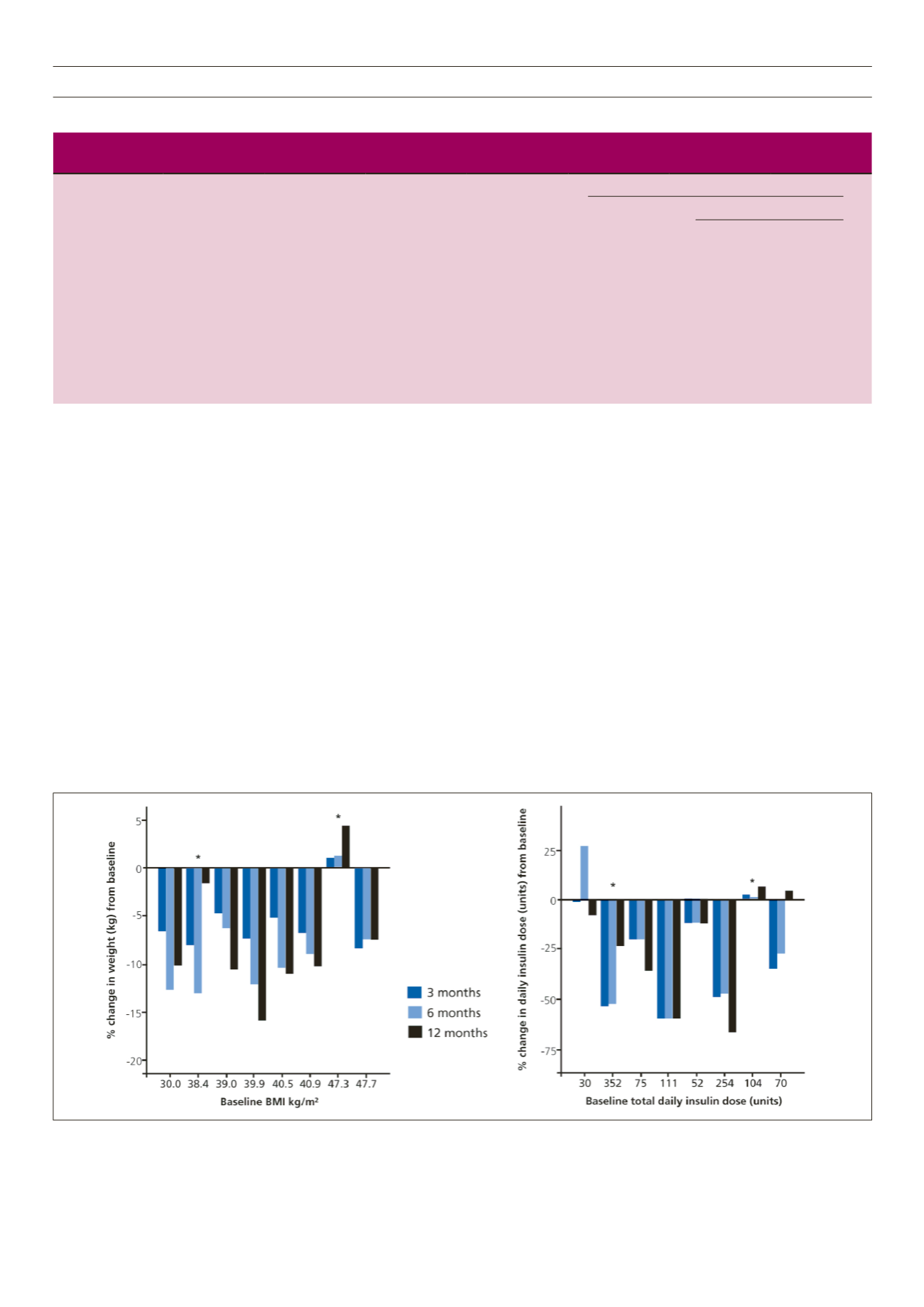
Figure 1.
Percentage changes in weight (left panel) and total daily insulin dosage (right panel) over three, six and 12 months compared to baseline in individual cases.
Relevant individual baseline parameters are shown on the abscissa and are in ascending BMI order. Cases that withdrew at six months are denoted by an asterisk.
Table 1.
Mean ± SD (range) of outcomes of HbA
1c
, weight and insulin dosage over time in patients with type 1 diabetes treated with GLP-1 agonist
therapy; p-values are for the Friedman test for repeated measures over time. Significance is set at 0.05.
p
-value
6 months
12 months
Baseline
3 months
6 months
12 months
n
= 8
n
= 8
n
= 6
HbA
1c
(%)
8.5 ± 1.7
(7.1–12.5)
8.4 ± 1.3
(7.0–11.2)
8.0 ± 0.9
(6.7–8.7)
8.3 ± 1.6
(6.5–12.0)
0.497
0.771
0.409
Weight (kg)
123.0 ± 23.9
(70.9–153.2)
116.2 ± 24.5
(66.2–154.8)
113.0 ± 25.9
(62.0–155.0)
114.1 ± 26.4
(63.8–160.0)
0.021
0.026
0.003
Insulin dose
(units/day)
131 ± 112
(30–352)
79 ± 49
(30–166)
81 ± 49
(38–168)
89 ± 78
(28–270)
0.045
0.107
0.044
Insulin dose
(units/kg/day)
1.0 ± 0.9
(0.4–2.9)
0.7 ± 0.4
(0.4–1.5)
0.7 ± 0.4
(0.4–1.6)
0.8 ± 0.6
(0.4–2.3)
0.044
0.136
0.158



















