SA JOURNAL OF DIABETES & VASCULAR DISEASE
DRUG TRENDS
VOLUME 10 NUMBER 1 • MARCH 2013
43
Table 1.
Antihyperglycaemic agents used in type 2 diabetes: sulphonylureas.
Class
Drug (brand name)
Effect on
HbA
1c
level
Therapeutic considerations
Disadvantages
Insulin
secretagogues
Sulphonylureas
Glibenclamide (Daonil
®
,
Euglucon
®
, generic)
Gliclazide (Diamicron
®
,
Diamicron MR 60 mg,
generic) Glimepiride
(Amaryl
®
, generic)
Glipizide (Minidiab
®
)
Generally well tolerated. Proven reduction
in microvascular endpoints (UKPDS and
ADVANCE studies); reduction in cardio-
vascular events and mortality in the long
term (UKPDS post-trial monitoring).
Relativelyrapidglucose-loweringresponse;
useful in the patient with symptomatic
hyperglycaemia. Consider using other
classes of antihyperglycaemic agents in
patients at high risk of hypoglycaemia
(e.g. the elderly, with renal and hepatic
failure). If a sulphonylurea must be used
in such individuals, gliclazide modified
release is associated with the lowest inci-
dence of hypoglycaemia. Glimepiride and
glipizide are associated with less hypogly-
caemia than glibenclamide.
Hypoglycaemia relatively common, but
variable. Can cause severe hypoglycae-
mia, including episodes necessitating
hospital admission and causing death
(particularly glibenclamide, and particu-
larly when renal function is impaired).
Causes weight gain (2–5 kg); worst with
glibenclamide. May blunt myocardial
ischaemic preconditioning (particularly
glibenclamide). Renal impairment: glib-
enclamide contraindicated if eGFR < 60
ml/min/1.73 m
2
; glimepiride and glipiz-
ide dose may need to be reduced
Second-generation sulphonylureas: gliclazide modified release (60 mg)
reviewed in clinical practice for type 2 diabetes
T
he modern use of sulphonylureas (SU)
in the management of type 2 diabetes
focuses on second-generation agents with
a lower risk of hypoglycaemia and weight
gain than older agents. This is particularly
important as the recently available oral
incretin mimetics offer modest HbA
1c
level
reduction with minimal or no hypoglycaemic
events and are either weight neutral or may
result in weight loss.
The comprehensive 2012 South African
Guidelines for the Management of Type 2
Diabetes,
1
compiled with the involvement
of clinicians, academic endocrinologists
and funders such as the Council of Medi-
cal Schemes and the Department of Health,
were published recently and stress lifestyle
modification, education and multi-discipli-
nary care. These guidelines advocate the
use of sulphonylureas at steps 1, 2 and 3
in combination with other oral antidiabetic
agents and insulin. At step 1, metformin is
the preferred therapy, with sulphonylureas,
DPP-4 inhibitors and acabose as alternative
therapies for special circumstances.
In their clinical review, the experts
compiling the South African Guidelines
summarised the role of the insulin secre-
tagogues and the therapeutic considera-
tions for their use on the basis of evidence
from long-term use in the UKPDS and the
ADVANCE trials (Table 1).
Review of gliclazide MR
In terms of the three above-mentioned dis-
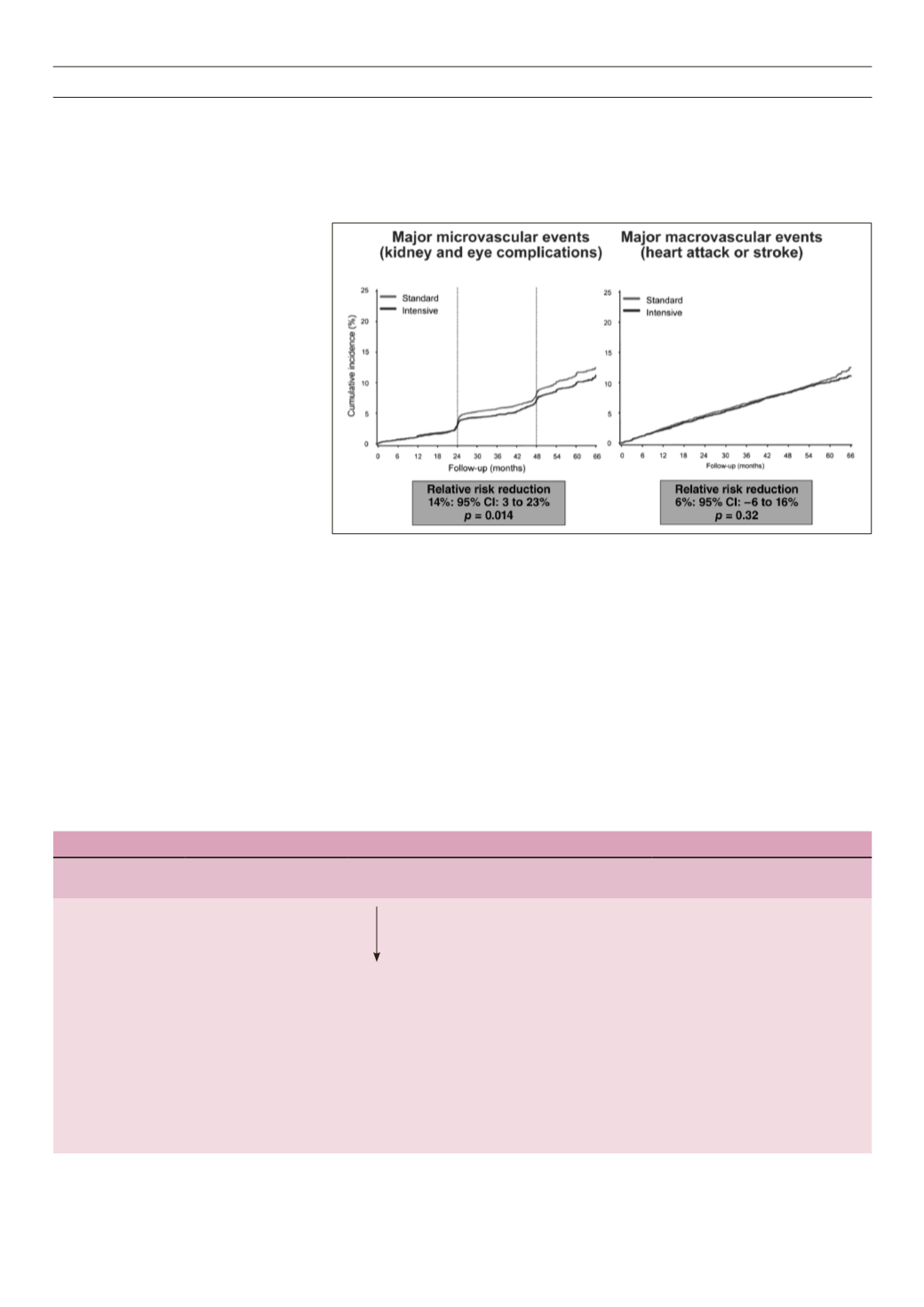
Fig. 1.
Micro- and macrovascular events in the ADVANCE study.
advantages of sulphonylureas: hypoglycae-
mia, weight gain and use in renally impaired
patients, the evidence for gliclazide MR
places it outside these general limitations.
Hypoglycaemia and gliclazide MR
The ADVANCE study used gliclazide modi-
fied release with metformin as the basis of
oral therapy for the intensive arm of this
important large study in type 2 diabetes
patients. At the end of the study, 90% of
patients were on Diamicron MR 60 mg, with
70% receiving a daily dosage of 120 mg
(2 tablets); 40% of patients were also on
insulin to intensify their treatment and
reach the targeted HbA
1c
level of less than
7%, and preferably below 6.5%.
2
The ADVANCE study achieved a 12%
reduction in cardiovascular death in the
intensive arm, showing a trend to benefit.
This is reassuring as there is no increase
in cardiovascular risk. The microvascular
outcomes were significant, with major
benefits for the kidney (Fig. 1).
In the intensive arm of ADVANCE,
hypoglycaemia was much lower than in the
intensive arms of either the ACCORD or the
VADT trials. In the 5 571 patients treated
in the intensive arm for a mean period of
4.9 years, only 150 hypoglycaemic events


