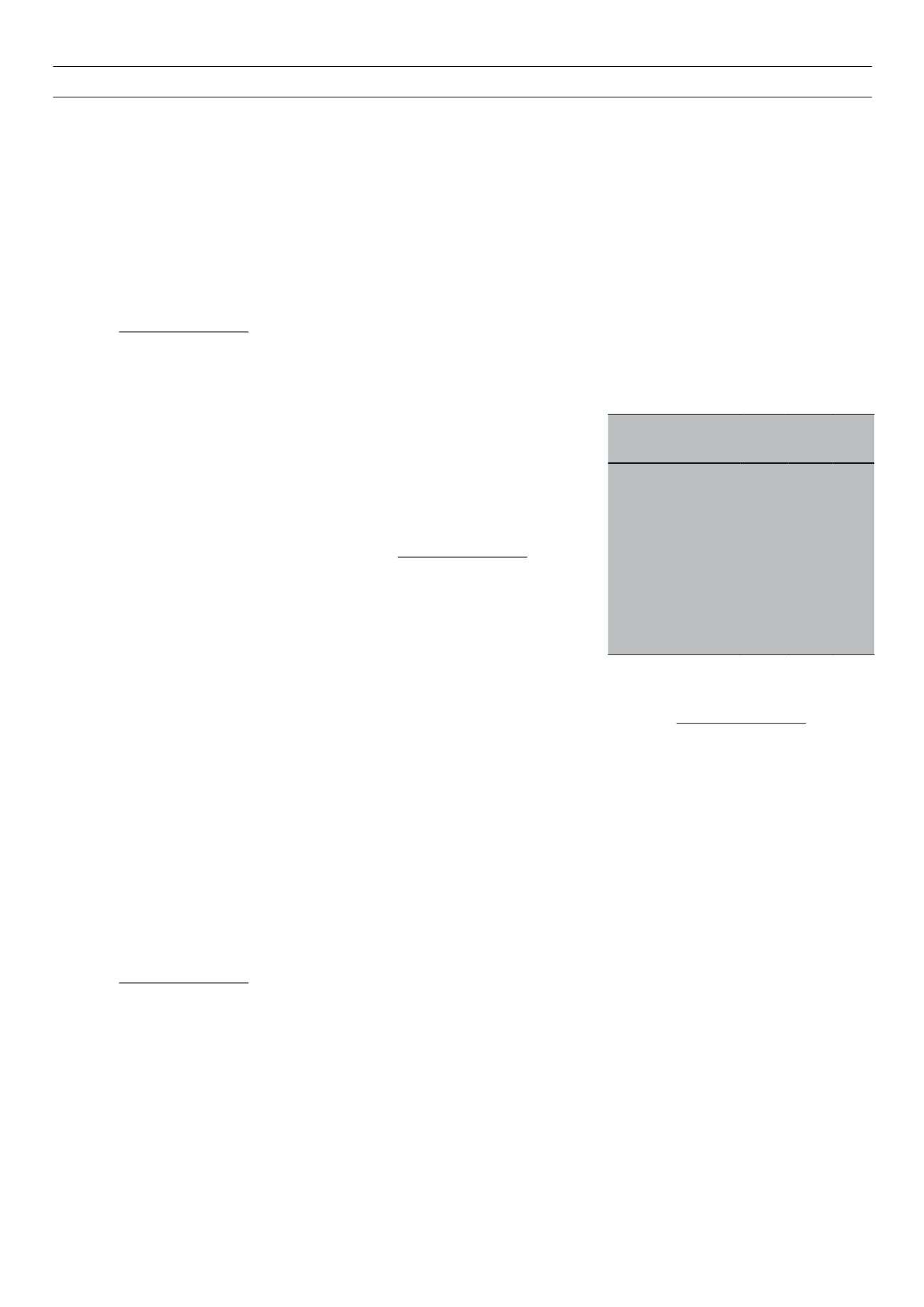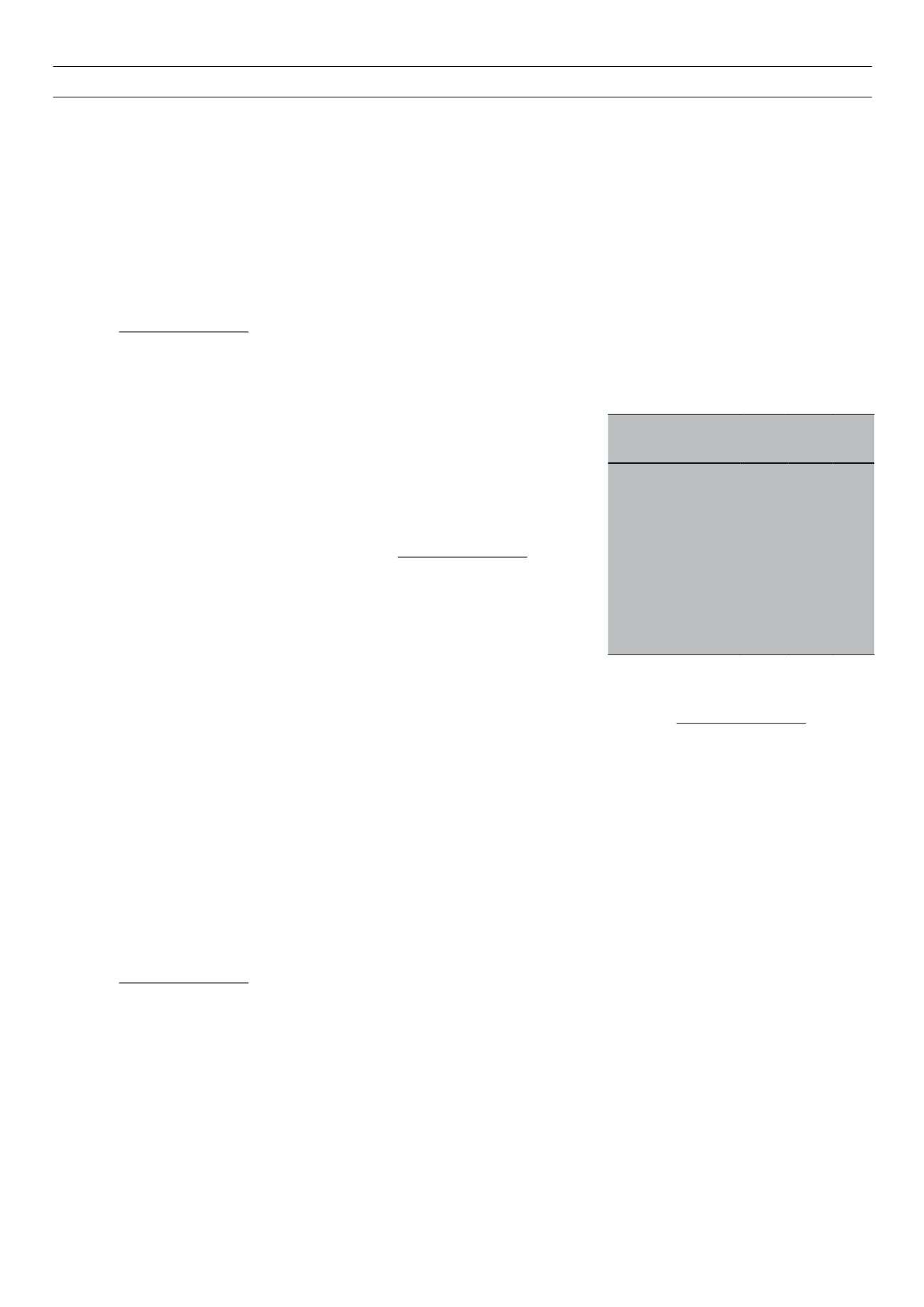
46
VOLUME 8 NUMBER 1 • MARCH 2011
SA JOURNAL OF DIABETES & VASCULAR DISEASE
JOURNAL UPDATE
Journal Update
Autoimmunity in a South African and African context
T
his review of the latest international jour-
nals focuses on the theme of autoimmunity
with the particular inclusion of recent articles
by South African researchers in the broader
field of diabetes care.
Autoimmunity, Addison’s disease and
accompanying autoimmune condi-
tions in a large South African cohort
In patients with Addison’s disease in South
Africa, accompanying autoimmune diseases
such as primary hypothyroidism (47%) pre-
mature ovarian failure (8%) and type 1 dia-
betes (7%) were the most prevalent.
This study
1
of 144 South African Addison’s
disease patients (94 of European descent,
34 mixed ancestry, five Asians and 11 Afri-
cans) recruited mainly from major centres
(often treated at teaching hospitals) showed
that autoimmunity accounts for at least
half of the patients with Addison’s disease.
The genetic assessment excluded patients
with autoimmune Addison’s disease and
associated autoimmune conditions, to elimi-
nate the bias these conditions can create
with HLA associations. The presence of type
1 diabetes was reportedly strongly associ-
ated with DQB1 *0201 and DQB1 *0302
alleles. When Addison’s patients with type
1 diabetes were removed from the evalua-
tion, an association with *0201 and *0302
remained.
HLA DQB1 *0201 alleles predominated
in the autoimmune group, but none of the
black Africans or Asians in this cohort had
adrenal antibodies. The authors noted the
limiting factor in this group was related to
small sample size and requires confirmation
in larger studies.
Source: Ross I, Boullet A, Soule S, Levitt N, Pirie F,
et al
.
Clin
Endocrinol
2010;
73
: 291–298. DOI: 10.1111/j.1365-
2265.2010.03807.x
Management of diabetes – South
African paediatric study of type 1
diabetic patients
In a comparative efficacy and safety study
of insulin glulisine and insulin lispro, given
as part of a basal-bolus insulin regimen
over 26-weeks to paediatric type 1 diabetic
patients, this Cape Town-managed study
showed both treatments were equally effec-
tive and similarly well tolerated. The study
included 572 children and adolescents (4–17
years old) using insulin glargine or neutral
protamine Hagedorn insulin as basal insulin,
who were enrolled to receive glulisine (
n
=
277) or lispro (
n
=
295) 0–15 min pre-meal.
The results showed similar baseline-to-
endpoint HbA
1c
changes. Overall, for all age
groups together, the percentage of patients
achieving American Diabetes Association
age-specific HbA
1c
targets at endpoint was
however significantly higher (
p
=
0.039)
with glulisine (38.4%) than lispro (32.0%).
Symptomatic hypoglycaemic rates were
similar.
Source: Philotheou A, Arslanian S, Blatniczky L, Peterkova
V, Souhami E, Danne T.
Diabetes Technol Ther
2011 Feb
3. Published ahead of print.
Malnutrition-related diabetes seen
in northern Ethiopia
A detailed clinical, biochemical and immu-
nological study of a group of ‘type 1 dia-
betic patients’ was undertaken at Mekelle
Hospital in northern Ethiopia, which serves
a poor rural area; 36% of the total group
(105 patients) had immunological or C-pep-
tide characteristics inconsistent with typical
type 1 or 2 diabetes. The clinical character-
istics, local prevalence of under-nutrition
and GADA and C-peptide heterogeneity are
suggestive of a malnutrition-related form of
diabetes.
Of the 105 patients, 74 (68%) were
men, mean age (
±
SD) was 41
±
16 years
and diabetes duration [median (interquartile
range)] was 5 (3–10) years. A family history
of diabetes was present in 10 (9%) patients;
median (interquartile range) BMI was 20.6
(18.5–23.9) kg/m
2
. Treatment was with diet
(
n
=
1), oral agents (
n
=
35) and insulin (
n
=
69). Only 5% of patients were hyperten-
sive (BP
>
140/80 mmHg). Glycaemic control
was poor with HbA
1c
high at 11.3
±
2.8%.
The failure of C-peptide to correlate
negatively and GADA positively is explored
further in Table 1, and led to the subdivision
of the group into: (1) ‘definite’ type 1 dia-
betes (GADA-positive, C-peptide-negative
and on insulin); (2) ‘definite’ type 2 diabetes
(GADA-negative, C-peptide-positive and on
any treatment); and (3) ‘uncertain’ (all other
patients).
These revised groupings gave a much
more expected proportion of type 1 dia-
betes patients at 17%, with type 2 diabe-
tes comprising 47% and the ‘uncertain’
group 36%. Patients in the latter group
were closer to type 1 than type 2 diabetes
in terms of age (current and at diagnosis),
BMI and HbA
1c
levels, with 89% on insulin,
29% GADA-positive and 71% C-peptide-
negative.
Table 1.
Interrelations between GADA and C-peptide
status in 105 Ethiopian diabetic patients.
Variable
Negative Positive p-value
C-peptide
Number of patients
45
60
GADA-positive,
n
(%)
10 (40) 11 (18) 0.04
On insulin,
n
(%)
42 (93) 27 (45) 0.0001
GADA
Number of patients
29
76
C-peptide-negative,
n
(%) 18 (62) 27 (35) 0.016
On insulin,
n
(%)
24 (83) 45 (59) 0.037
Source: Gill GV. Tekle A, Reja A, Wile D, English PJ,
et
al
.
Diabetologia
2011;
54
: 51–57. DOI: 10.1007/s00125-
010-1921.
Traditional root bark from tree
(
Euclea undulata
) in Limpopo prov-
ince shows hypoglycaemic activity
In this study from the Department of Plant
Science, University of Pretoria, an acetone
plant extract from
Euclea undulata
var.
myr-
tina
(Ebenaceae) used by traditional healers
was tested for hypoglycaemic activity on
myocytes and ability to inhibit L-glucosidase.
The positive results obtained from the
in vitro
assays on the main fractions with
myocytes (C2C12), pre-adipocytes (3T3-L1)
and chang liver cells led to the isolation of
four compounds for further testing. These
compounds were L-amyrin-30
β
-(5-hydroxy)
ferulic acid, lupeol, betulin and epicatechin.
These compounds were then assessed
for their ability to inhibit the carbohydrate-
hydrolising enzyme L-glucosidase. The feru-
lic acid was shown to inhibit L-glucosidase,
while epicatechin had some effects
in vitro
on the cell lines to lower blood glucose levels.
Source: Deutschlander MS, Lall N, van de Venter M,
Hussein AA.
J Ethnopharmacol
2011;
133
: 1091–1095.