
SA JOURNAL OF DIABETES & VASCULAR DISEASE
RESEARCH ARTICLE
VOLUME 13 NUMBER 2 • DECEMBER 2016
79
may be caused by micro-angiopathy or endothelial dysfunction.
Accordingly, it reflects an increased likelihood of future coronary
events.
21
The majority of studies on ischaemia have used SPECT MPI. An
analysis of the diagnostic accuracy of pharmacologically induced
stress MPI reported a mean sensitivity and specificity of 88 and
77%, respectively.
22
Platelet volume is a marker of platelet activation and function,
and is measured using MPV.5 Platelets that have dense granules
are more active biochemically, functionally and metabolically. Large
platelets secrete high levels of prothrombogenic thromboxane A2,
serotonin, beta-thromboglobulin and procoagulant membrane
proteins such as P-selectin and glycoprotein IIIa.
5,23
Platelets
secrete a large number of substances that are crucial mediators of
coagulation, inflammation, thrombosis and atherosclerosis.
24,25
It is
also well known that large platelets are a risk factor for developing
coronary thrombosis, leading to myocardial infarction.
19,23,26,27
Measurement of platelet activation and/or aggregation may
provide prognostic information in patients at risk for or following
a cardiovascular event.
28,29
Reports have revealed that there is a
close relationship between MPV and cardiovascular risk factors,
including impaired fasting glucose levels, diabetes mellitus,
hypertension, hypercholesterolaemia, obesity and the metabolic
syndrome.
30-32
Increased platelet activity is reported to play a role in
the development of vascular complications in diabetic patients.
18
MPV was increased in patients with SCF complex and cardiac
syndrome X, both being related to microvascular defects and
endothelial dysfunction.
33,34
In the present study, we showed that
MPV was associated with myocardial perfusion defect, using MPI
in diabetic patients.
In our study, MPV was increased in the myocardial perfusion
defect group compared to those without myocardial perfusion
defects. DM not only involves the main coronary artery but also the
microvascular circulation, leading to myocardial perfusion defects.
Perfusion defects are significant predictors of coronary events in
patients with known or suspected CHD.
20
The main limitation of our study was the small sample size, which
could result in low statistical power for equivalency testing, leading
to false-negative results. Second, because of the retrospective
nature of data collection, the angiographic results of the patients
were not evaluated. MPI may reflect myocardial perfusion defects
but it was not able to show the anatomical status of the coronary
artery. We cannot extend our results to the general population due
to our broad exclusion criteria.
Conclusion
MPV levels were higher in the diabetic patients with myocardial
perfusion defects than in those without myocardial perfusion
defects. In diabetic patients, increased MPV may be an independent
marker of myocardial perfusion defects, which are associated with
adverse coronary events.
References
1.
Whiteley L, Padmanabhan S, Hole D, Isles C. Should diabetes be considered a
coronary heart disease risk equivalent? results from 25 years of follow-up in the
Renfrew and Paisley survey.
Diabetes Care
2005;
28
: 1588-1593.
2.
Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham
study.
J Am Med Assoc
1979;
241
: 2035-2038.
3. Nathan DM, Meigs J, Singer DE. The epidemiology of cardiovascular disease in
type 2 diabetes mellitus: how sweet it is ... or is it?
Lancet
1997;
350
(Suppl 1):
SI4-9.
4.
Misko J. Evaluation of myocardial perfusion and viability in coronary artery disease
in view of the new revascularization guidelines.
Nuclear Med Rev Central Eastern
Eur
2012;
15
: 46-51.
5.
Martin JF, Shaw T, Heggie J, Penington DG. Measurement of the density of human
platelets and its relationship to volume. Br J Haematol 1983; 54: 337-352.
6.
Erhart S, Beer JH, Reinhart WH. Influence of aspirin on platelet count and volume
in humans.
Acta Haematol
1999;
101
: 140-144.
7. Zuberi BF, Akhtar N, Afsar S. Comparison of mean platelet volume in patients with
diabetes mellitus, impaired fasting glucose and non-diabetic subjects.
Singapore
Med J
2008;
49
: 114-116.
8. Cerqueira MD, Weissman NJ, Dilsizian V,
et al
. Standardized myocardial
segmentation and nomenclature for tomographic imaging of the heart. A
statement for healthcare professionals from the Cardiac Imaging Committee of
the Council on Clinical Cardiology of the American Heart Association.
Circulation
2002;
105
: 539-542.
9. Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: the Michigan method
for quantitative nuclear cardiology.
J Nucl Cardiol
2007;
14
: 455-465.
10. Murea M, Ma L, Freedman BI. Genetic and environmental factors associated with
type 2 diabetes and diabetic vascular complications.
Rev Diabet Studies
2012;
9
:
6-22.
11. Bae SH, Lee J, Roh KH, Kim J. Platelet activation in patients with diabetic
retinopathy. Korean J Ophthalmol 2003; 17: 140-144. 12. Brownlee M.
Biochemistry and molecular cell biology of diabetic complications.
Nature
2001;
414
: 813-820.
13. Kitada S, Otsuka Y, Kokubu N,
et al
. Post-load hyperglycemia as an important
predictor of long-term adverse cardiac events after acute myocardial infarction: a
scientific study.
Cardiovasc Diabetol
2010;
9
: 75.
14. Nishimura R, Nakagami T, Sone H, Ohashi Y, Tajima N. Relationship
between hemoglobin A1c and cardiovascular disease in mild-to-moderate
hypercholesterolemic Japanese individuals: subanalysis of a large-scale
randomized controlled trial.
Cardiovasc Diabetol
2011;
10
: 58.
15. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death
in the WHO Multinational Study of Vascular Disease in Diabetes.
Diabetologia
2001;
44
(Suppl 2): S14-21.
16. Koistinen MJ. Prevalence of asymptomatic myocardial ischaemia in diabetic
subjects.
Br Med J
1990;
301
: 92-95.
17. Prevalence of unrecognized silent myocardial ischemia and its association with
atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Milan
Study on Atherosclerosis and Diabetes (MiSAD) Group.
Am J Cardiol
1997;
79
:
134-139.
18. Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship
between glycemic control and platelet activity in type 2 diabetes mellitus.
J
Diabetes Complicat
2009;
23
: 89-94.
19. Senaran H, Ileri M, Altinbas A,
et al
. Thrombopoietin and mean platelet volume
in coronary artery disease.
Clin Cardiol
2001;
24
: 405-408.
20. Thomas GS, Miyamoto MI, Morello AP, 3rd,
et al
. Technetium 99m sestamibi
myocardial perfusion imaging predicts clinical outcome in the community
outpatient setting. The Nuclear Utility in the Community (NUC) Study.
J Am Coll
Cardiol
2004;
43
: 213-223.
21. Nitenberg A, Ledoux S, Valensi P, Sachs R, Attali JR, Antony I. Impairment of
coronary microvascular dilation in response to cold pressor-induced sympathetic
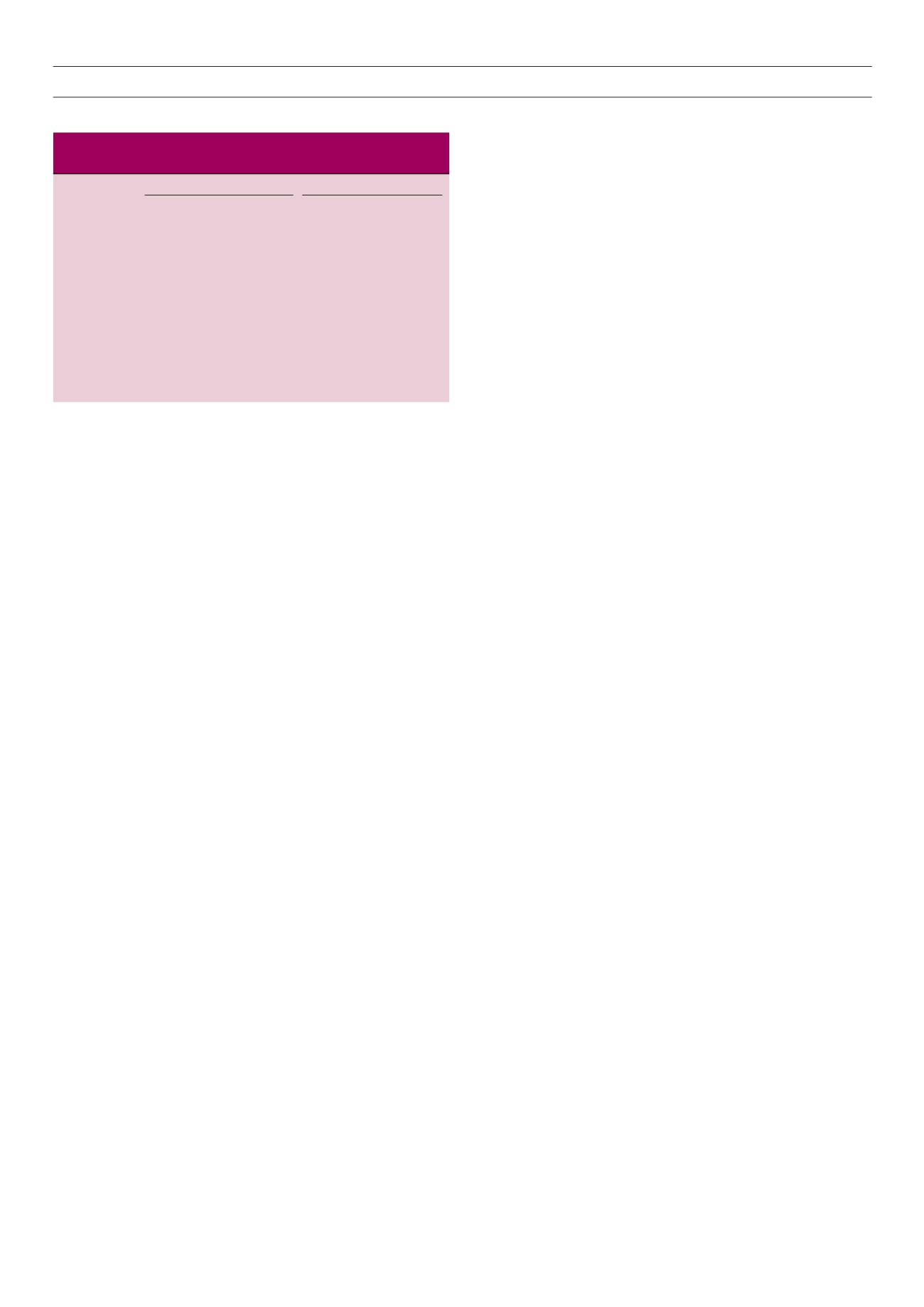
Table 2.
Univariate and multivariate regression analyses of
independent variables for MPD.
Univariate Multivariate
Variables
OR 95% CI
p
-value OR 95% CI
p
-value
MPV (fl)
2.401 1.298–4.440 0.005 2.484 1.215–5.081 0.013
Glucose (mg/dl) 1.009 0.999–1.029 0.072 1.008 0.997–1.019 0.178
HbA
1c
(%)
1.800 0.993–3.474 0.08 1.984 0.980–4.018 0.064
Age (years)
1.011 0.963–1.061 0.664
Gender
1.244 0.497–3.16 0.641
HT (mg/dl)
2.375 0.801–7.043 0.119
BMI (km/m2)
0.991 0.92–1.067 0.820
TC (mg/dl)
0.994 0.984–1.004 0.256
TG (mg/dl)
0.998 0.994–1.002 0.360
HDL-C (mg/dl) 0.948 0.878–1.023 0.167
LDL (mg/dl)
0.989 0.975–1.003 0.134
Hb (%)
1.138 0.845–1.534 0.395



















