SA JOURNAL OF DIABETES & VASCULAR DISEASE
ACHIEVING BEST PRACTICE
VOLUME 7 NUMBER 4 • NOVEMBER 2010
161
In-practice experience in South Africa
We have had experience in using exenatide under MCC Section 21
conditions in 13 T2DM patients. These are observational data but do
give us some insight into the South African situation. After the drug
was registered, the MCC did not initially allow distribution, as the
pricing committee had not finalised the cost of the two strengths of
the drug. Patients therefore had a four- to five-month period where
exenatide could not be obtained. This gave us a significant window
to assess the effect of drug withdrawal on patients.
Of the 13 patients on exenatide, all used the drug at 5 mcg bd
for four weeks, then increased to 10 mcg bd. All patients showed
significant improvements in post-prandial sugar levels and HbA
1c
control, with no hypoglycaemic events, and several experienced
weight loss with this agent. One patient had to be withdrawn, as
he did not feel it benefited him and he was nauseous with the
drug, even at a lowered dosage. He was not part of the 13-patient
cohort.
The best weight reduction in a male patient was 15 kg. He had a
significant reduction in insulin dosages, to the point where he only
required a third of the original insulin dosage. When the exenatide
was stopped, his glucose control deteriorated rapidly, as did his
weight control. Most of these patients were on a combination of
metformin plus insulin, usually a basal-bolus regimen and had a
BMI in excess of 35 kg/m
2
.
With some patients we did not have a follow-up HbA
1c
value
before the exenatide became unavailable, but patients reported
feeling better, with improved glucose levels, on home monitoring.
There were two patients, both difficult to control normally, who did
not find a significant benefit with exenatide. It is uncertain whether
a higher dosage could have been useful in these patients.
The best result was in a 52-year-old lady who was diabetic for
10 years on basal-bolus insulin. Her BMI was 59 kg/m
2
and she
had a total weight loss of 35 kg. Her HbA
1c
dropped to 8.2% on
treatment but deteriorated to 10.9% once exenatide became
unavailable. One patient with a weight loss of 10 kg stopped her
short-acting insulin completely as she was close to hypoglycaemic
values, and she was able to reduce her basal insulin to 10 units
where she had previously required 44 units/day.
Conclusion
In a review ‘Pharmacotherapy in diabetes: When do we use a new
drug?’, Sherwin says the following: ‘The advantages of the GLP-1
agonists are weight loss, low rate of hypoglycaemia, may preserve
β
-cell mass, reduce post-prandial glucose, but have gastro-intestinal
side effects, has a high cost, is an injection and we have less long-
term safety data’.
5
Exenatide was approved by the FDA in 2005
and apart from the known side effects, nothing more has surfaced.
Pancreatitis is a possible complication of diabetes
per se
, and
gallstone disease is more prevalent in diabetic patients. The risk
of pancreatitis must be explained to patients and they should be
warned about possible signs and symptoms in order to effect quick
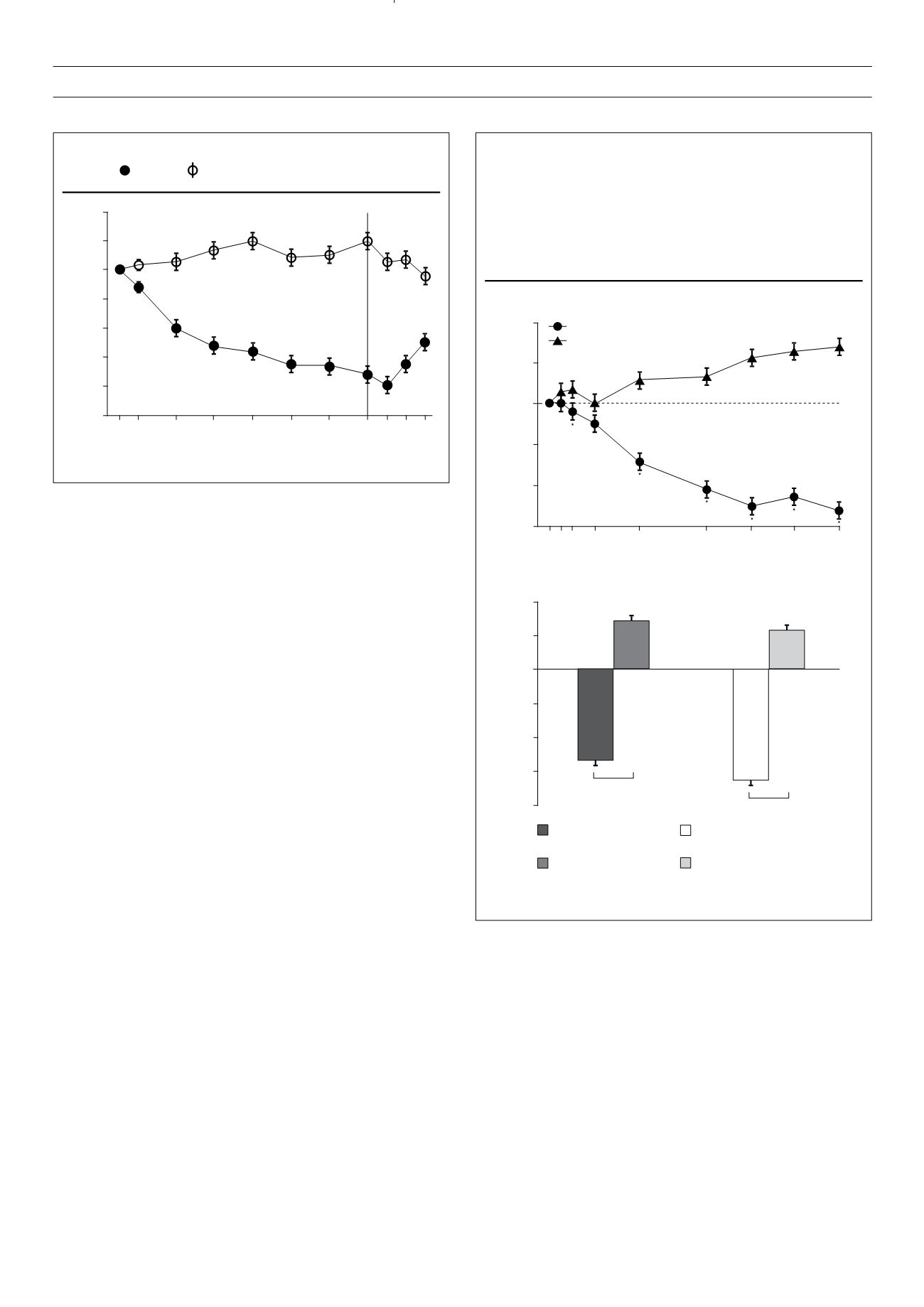
Figure 3.
Mean changes in body weight in patients receiving exenatide
once weekly or insulin glargine titrated to target.
(A) Mean changes in body weight with time. Baseline body
weights were 91.2 kg (SE 1.2) for patients taking exenatide
and 90.6 kg (1.2) for those taking insulin glargine. (B) Mean
changes in body weight for all patients versus patients receiving
study drug plus metformin only. Error bars show standard error.
*Between-group difference was significant (
p
<
0.05).
Least-squares mean change in body weight (kg)
Exenatide once weekly (
n
=
233)
Insulin glargine (
n
=
222)
Time (weeks)
2
1
0
–1
–2
–3
0 1 2 4
8
14 18 22 26
A
Least-squares mean change in body weight (kg)
2
1
0
–1
–2
–3
–4
B
Study drug plus metformin only
Overall
–4.0 (95% CI, –4.6 to –3.5)
–4.4 (95% CI, –4.6 to –3.6)
Exenatide once weekly
(
n
=
233)
Insulin glargine
(
n
=
222)
Exenatide
+
metformin only
(
n
=
164)
Insulin glargine
+
metformin only
(
n
=
157)
Figure 2.
Changes in body weight over 52 weeks of treatment.
exenatide, insulin glargine.
Change in weight (kg)
Weeks
2
1
0
–1
–2
–3
–4
–5
0 4 12 20 28 36 44 52 56 60 64
Adapted from Bunk
et al. Diabetes Care
2009;
32
: 762–768.
Adapted from
Lancet
2010;
375
(9733): 2234–2243.


