
VOLUME 12 NUMBER 1 • JULY 2015
23
SA JOURNAL OF DIABETES & VASCULAR DISEASE
RESEARCH ARTICLE
and 160 mg/day), with potency scores ranging from 1 (5 mg/day
simvastatin) to 6 (160 mg/day simvastatin).
23,27
The 2011 ESC guidelines were used to classify CV risk, LDL-C level
treatment goals, and sub-optimal HDL-C and triglyceride levels.
21,28
Variables independently associatedwith dyslipidaemiawere evaluated
with logistic regressionmodelling using the following variables: age (≥
70 years), female gender, family history of premature coronary heart
disease (CHD), current tobacco smoker, sedentary lifestyle, alcohol
consumption (> 2 units/week), body mass index (BMI) ≥ 30 kg/m
2
,
large waist circumference (> 102 cm in men, > 88 cm in women
29
),
hypertension, DM, coronary heart disease, cerebrovascular disease,
heart failure, peripheral artery disease, systolic/diastolic blood
pressure ≥ 140/90 mmHg, simvastatin equivalent dose of either 20
to 40 versus 10 mg/day, or > 40 mg versus 10 mg/day, ezetimibe use,
and physician’s specialty (cardiologist, endocrinologist, diabetologist,
internal medicine or other).
Statistical analysis
To estimate the sample size needed for South Africa we assumed a
prevalence of residual lipid abnormalities between 20 and 60% in
patients fulfilling the entry criteria for this study and a design effect
of 20% (variance inflation due to cluster sampling design). We
calculated that, within this range, a sample size of 1 000 would be
sufficient to estimate the prevalence of residual dyslipidaemia with
a given precision of ± 3.4% (range of 95% confidence interval:
6.8%). Furthermore we determined that this size guaranteed
enough information for estimating the prevalence in smaller
subgroups (representing one-quarter or more of the population)
with a precision of ± 6.8% (95% CI: 13.6%).
Following data collection, patient information was entered into a
central web-based database housed and managed at the Institut für
Herzinfarktforschung, Ludwigshafen, Germany. Real-time quality
control (internal logic checks) occurred during web-based data
entry. Continuous variables are presented as means with standard
deviations or medians with 25th and 75th percentiles [interquartile
range (IQR)] as indicated, and categorical variables are reported as
absolute numbers and percentages.
Kernel density estimation was used to analyse the distribution
of total cholesterol, LDL-C, HDL-C and triglyceride levels. The value
of a kernel density and its slope at the lipid value equal to the ESC
goal provides a crude indicator of the change in the proportions of
patients meeting the goal froma small improvement or deterioration
in lipid level starting from the ESC goal. This approach thus provides
a sensitivity analysis for either changes in the ESC goals or changes
in lipid levels for people whose levels are near the goals.
Multiple logistic regression analyses with backward selection (
α
= 0.05) were used to identify variables independently associated
with LDL-C, HDL-C and triglyceride irregularities. Two-tailed
statistical comparisons were used (
p
< 0.05 was significant) and
patients lacking the appropriate lipid parameters were not included
within the analyses. All analyses were performed using SAS v 9.1
(SAS Institute Inc, USA).
Results
Patient characteristics, risk categories and lipid parameters are
presented in Table 1. The study enrolled 1 029 patients (429 men,
600 women). The mean age of patients was 65.4 years, and 58.3%
were female. The study population was of mixed ethnic (multi-
racial) origin, including Caucasians (56.6%), blacks (22.0%), Asians
(9.5%) and patients of mixed ancestry (12.0%).
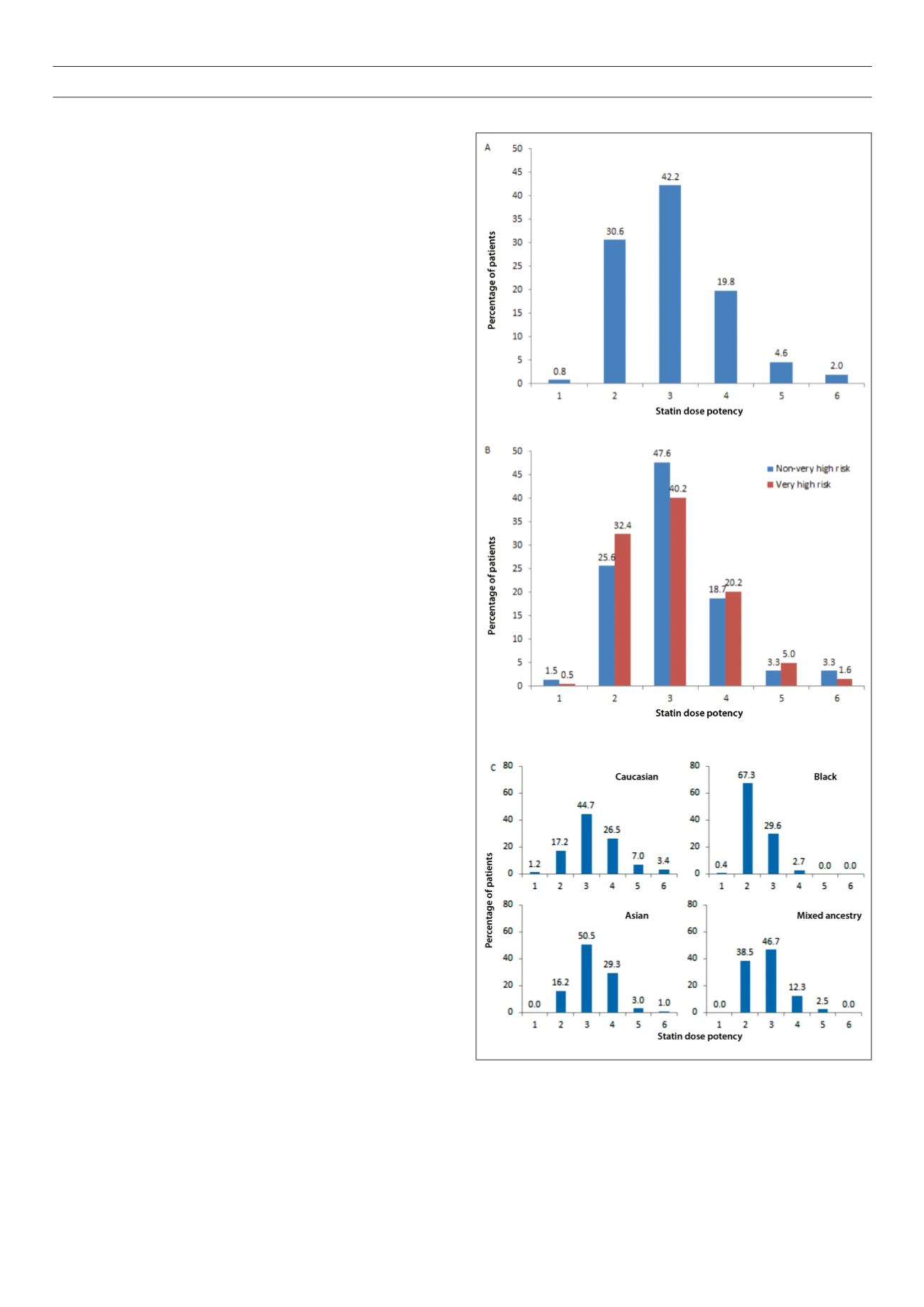
Fig. 1.
Statin dose potency overall (A), according to patients’ risk status (B), and
by ethnicity (C) calculated according to references 22, 23. *Statin dose potency
1 is equivalent to simvastatin 5 mg/day, potency 2 is equivalent to simvastatin
10 mg/day, potency 3 is equivalent to simvastatin 20 mg/day, potency 4 is
equivalent to simvastatin 40 mg/day, potency 5 is equivalent to simvastatin 80
mg/day, and potency 6 is equivalent to simvastatin ≥ 160 mg/day.



















