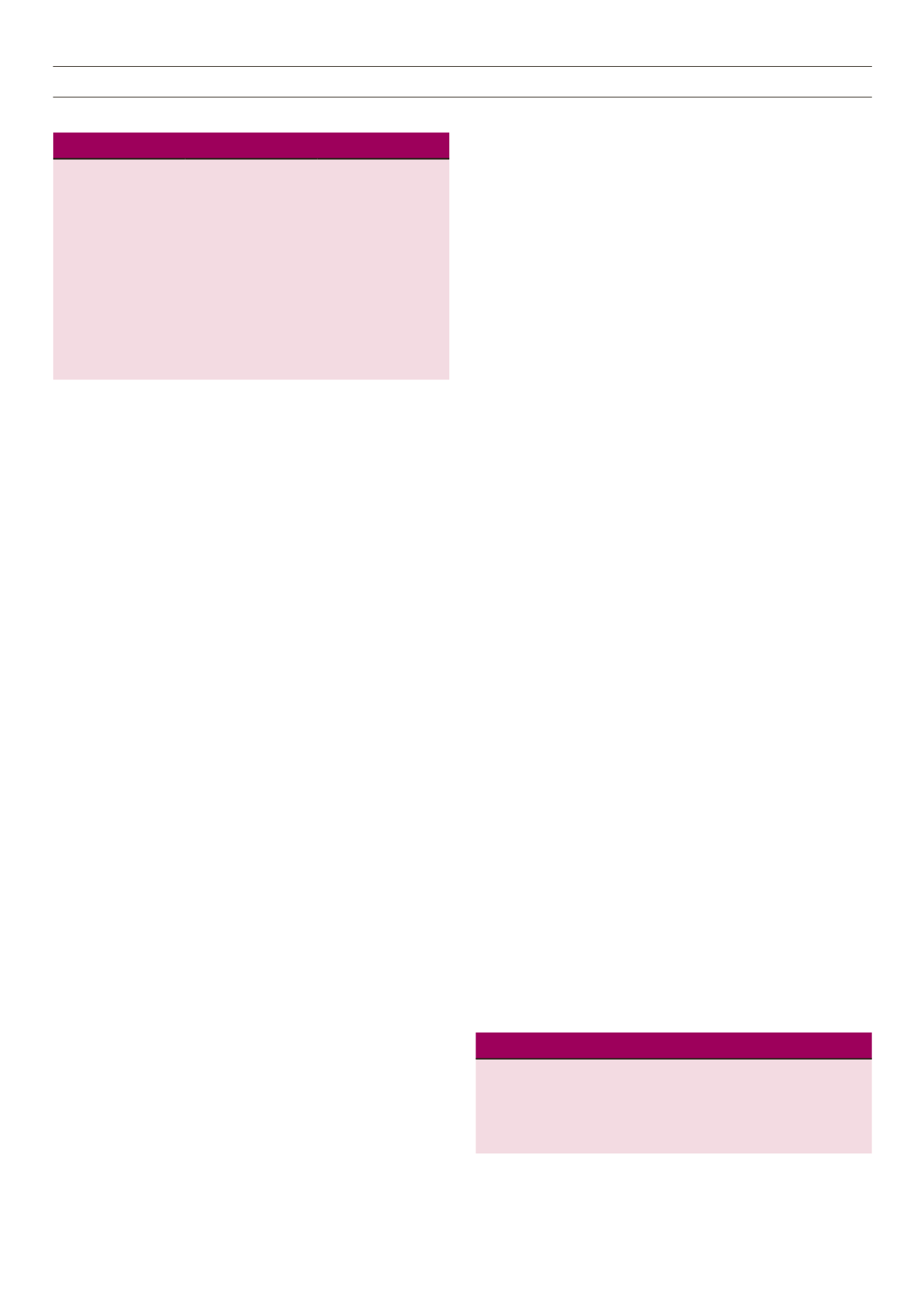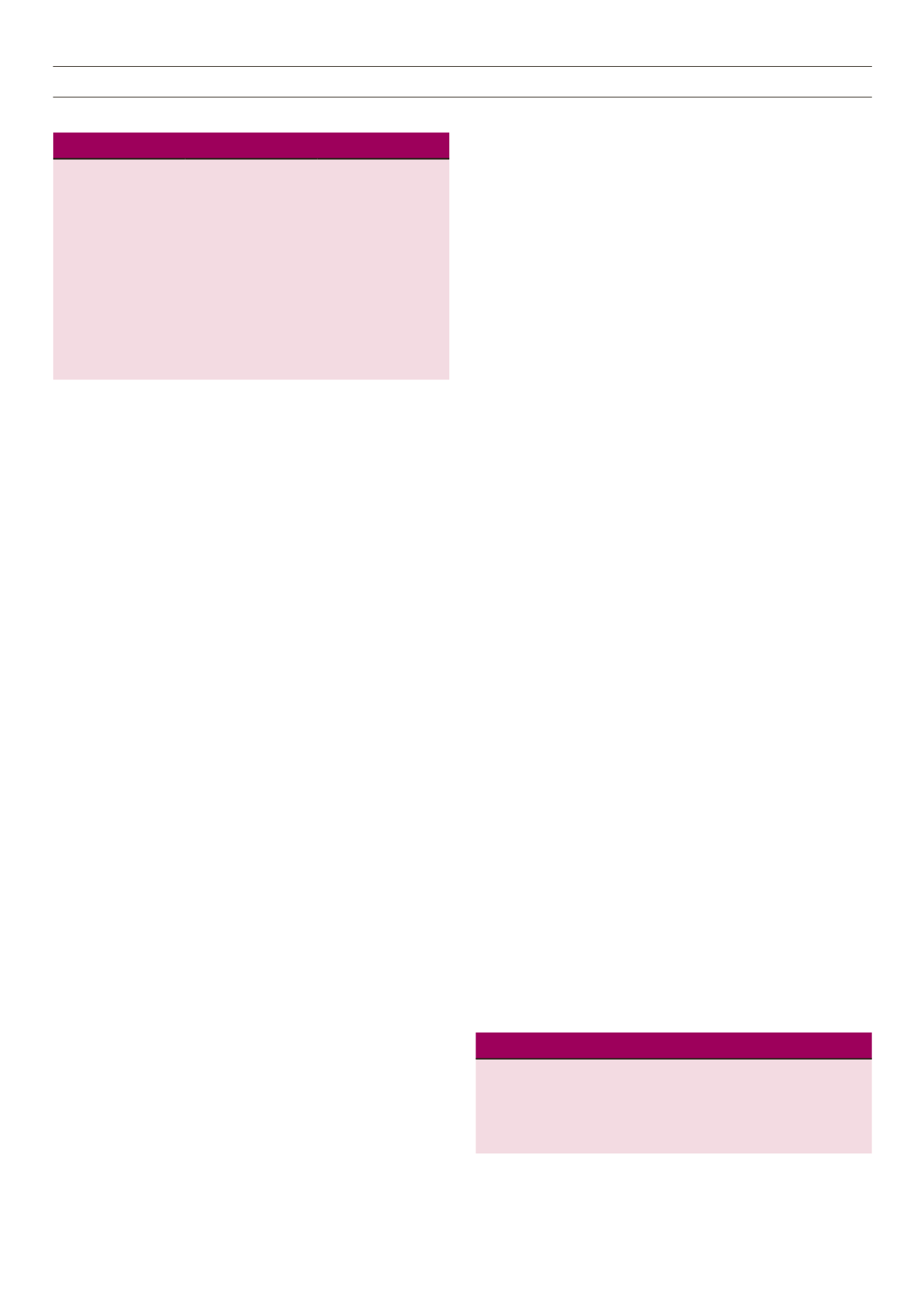
10
VOLUME 11 NUMBER 1 • MARCH 2014
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
In patients with diabetes, normal glucose thresholds are
disturbed, because glucose remains high when there is not strict
control, and low, with more frequent episodes of hypoglycaemia,
when treatment is more aggressive. Repeated hypoglycaemia
appears to reduce the glycaemic threshold to falling glucose
levels, so that there is a loss of both first- and second-line counter-
regulatory defences against an excessive drop in glucose levels.
Insulin levels remain high consequent to exogenous administration
and continual absorption from subcutaneous tissue where it was
injected.
Due, at least in part, to the deficiency of endogenous insulin
secretion secondary to beta-cell destruction, paracrine control
of pancreatic alpha-cells is lost and glucagon secretion remains
deficient, leaving the patient reliant on the sympatheto-adrenal
response to correct glucose levels. However, especially in patients
with strict glucose control, because the glycaemic threshold for
the adrenaline response has moved to a lower level (which may
be induced by recent hypoglycaemic episodes, exercise and sleep),
adrenaline secretion is reduced and delayed. Therefore all three of
the initial defences against hypoglycaemia (insulin, glucagon and
adrenergic responses) are impaired.
In addition to the detrimental effect on recovery from
hypoglycaemia, the insufficient sympatho-adrenergic response is
associated with decreased or absent symptoms that would normally
alert the patient to impending hypoglycaemia and provoke behaviours
to correct it. Consequently this unawareness to hypoglycaemia
increases the risk for severe iatrogenic hypoglycaemia.
1,4,11-13
In patients with type 2 diabetes and residual beta-cell function,
counter-regulatory mechanisms initially remain relatively intact,
sparing them from severe hypoglycaemia. However, as the disease
progresses, leading to failure of oral therapy and progression to
insulin injections, hypoglycaemic episodes become more frequent
and severe, and patients present with the same deficiencies in
counter-regulation that are experienced by type 1 diabetes patients.
Loss of awareness to hypoglycaemia may be complete or
partial. In partial unawareness, the symptom profile changes, with
reduction in either the intensity or number of symptoms over time.
However, even in aware diabetic patients, hypoglycaemic episodes
that do not elicit symptoms are frequently observable.
14
In a survey
of 372 outpatients with type 1 diabetes of mean duration 24
years, almost half had partial impairment of awareness, and in the
Diabetes Control and Complications Trial (DCCT), 36% of all severe
hypoglycaemia symptoms in awake patients were unaccompanied
by warning symptoms.
1,3,15
In 122 insulin-dependent type 2 diabetes
patients with a mean duration of diabetes of 15 years and insulin
therapy for six years, the prevalence of impaired awareness of
hypoglycaemia was approximately 10%.
16
In type 2 diabetes, hypoglycaemia unawareness is the most
important risk factor for severe hypoglycaemia.
17,18
Indeed, it
increases the incidence of severe hypoglycaemia six-fold in type 1
diabetes subjects and 17-fold in patients with type 2 diabetes.
16,19
In a study of 302 insulin-treated diabetic patients, 16% of whom
had partial awareness and 7% no awareness, 91% with absent
awareness and 69% with partial awareness had experienced severe
hypoglycaemia over the past year, compared with only 18% in
those whose awareness was normal.
20
Because it is episodes of hypoglycaemia itself that appear to
be the underlying cause for both defective counter-regulation of
glucose and unawareness of falling glucose levels, the syndrome
has been termed hypoglycaemia-associated autonomic failure
(HAAF). HAAF represents a vicious circle in which hypoglycaemic
episodes promote further episodes and hypoglycaemia becomes
recurrent.
4,21,22
The complex pathogenesis and theories regarding
the central nervous system-mediated mechanisms underlying HAAF
have recently been reviewed in detail by Cryer.
13
Although the glucagon response does not recover, avoidance
of hypoglycaemia for two to three weeks significantly improves
the adrenaline response and restores awareness of hypoglycaemia.
Therefore, avoidance of hypoglycaemia, and in particular nocturnal
episodes, should be the mainstay of prevention and treatment for
the hypoglycaemia unawareness syndrome.
11
Diagnosis of hypoglycaemia unawareness
If episodes of hypoglycaemia are to be prevented it is essential to
identify patients at risk. Table 3 lists some identifiable risk factors
for hypoglycaemia and unawareness.
In the absence of specific tests to establish the presence of
hypoglycaemia unawareness or defects in the glucose counter-
regulatory responses, diagnosis depends on careful history taking
from patients and family members to elicit unrecognised symptoms
or clues to hypoglycaemic episodes, and results from regular
blood glucose monitoring. Loss of hypoglycaemia awareness is
progressive and often incomplete and symptoms of hypoglycaemia
may change in number, nature and severity over time. Therefore
comparison with earlier episodes may be useful.
Timing of severe hypoglycaemia may also differ depending
on awareness of symptoms. In comparison with patients with
normal awareness who experienced a greater proportion of severe
hypoglycaemic episodes in the early morning, episodes were more
common during the evening and before retiring to bed in unaware
individuals and were unrelated to insulin administration.
23
Although diabetic neuropathy is associated with many of the
counter-regulatory defects that are present in patients with HA,
patients with HA frequently have no signs of either autonomic
neuropathy or microangiopathy.
1,20,24
Table 2.
Symptoms and signs of hypoglycaemia.
1,4
Autonomic
Neuroglycopenic
Physical signs
Sweating
Tremor
Feeling shaky
Feeling hungry
Palpitations
Anxiety
Feelings of warmth,
weakness and fatigue
Feeling faint or drowsy
Blurred vision
Difficulty speaking
Behavioural changes
Emotional lability and
irritability
Difficulty thinking
Confusion
Dizziness
Seizures
Coma
Pallor
Diaphoresis
Increased heart rate
Increased systolic
blood pressure
Table 3.
Risk factors associated with hypoglycaemia unawareness.
1,3,19
Antecedent or recurrent hypoglycaemia
Older age
Longer duration of diabetes
Lower HbA
1c
levels (good glycaemic control)
C-peptide negativity