
RESEARCH ARTICLE
SA JOURNAL OF DIABETES & VASCULAR DISEASE
36
VOLUME 16 NUMBER 1 • JULY 2019
attending the Ukwala HIV clinic were included. Those who declined
consent for the cardiovascular risk-factor screening and pregnant
women were excluded.
Patients fulfilling national eligibility criteria (CD4 count > 350
cells/mm
3
at time of the study) were treated with standard ART
according to national guidelines.
9
Standard regimens at that time
included tenofovir, lamivudine and efavirenz (TNF/3TC/EFV) or
zidovudine, lamivudine and efavirenz (AZT/3TC/EFV). Some were
still receiving stavudine, lamivudine and efavirenz (D4T/3TC/EFV),
which was being phased out at the time. A minority received a
lopinavir/ritonavir (LPV/r)-containing regimen.
Prior to commencing CVD screening within the HIV clinics
at Ukwala sub-county hospital, healthcare providers (including
nurses, laboratory technologists, clinicians and data clerks) in the
health facility received a two-day training, followed by regular
intensive theoretical and practical skills training and mentoring in
measuring and interpreting cardiovascular risk factors. The facility
was also provided with regularly calibrated point-of-care diagnostic
equipment for cardiovascular risk assessment.
Blood pressure (BP) was measured using a hospital-grade Omron
M3
®
(Omron, Netherlands) digital automaticbloodpressuremachine.
Hypertension diagnosis was based on standard guidelines, and
included blood pressure measurements, medical history, physical
examination, assessment of absolute cardiovascular risks (where
deemed necessary by the examining physician) and laboratory
investigations. A comprehensive assessment of BP involved multiple
measurements taken on separate occasions, at least twice or three
times, one or more weeks apart or sooner if the hypertension was
severe.
Hypertension was defined as per the seventh report of the
Joint National Committee on Prevention, Detection, Evaluation
and Treatment of High Blood Pressure (JNC 7)10 as follows: pre-
hypertension: systolic 120–139 mmHg, diastolic 80–89 mmHg;
stage 1 hypertension: systolic 140–159 mmHg, diastolic 90–99
mmHg; stage 2 hypertension: systolic ≥ 160 mmHg, diastolic ≥ 100
mmHg, and those currently on antihypertensive drugs.
Total cholesterol and blood glucose levels were measured in
the clinic using finger-prick blood by a Humansence
®
(Human,
Wiesbaden, Germany) meter calibrated with a control strip on
the first and after every 10th specimen. Raised total cholesterol
level was defined according to US National Cholesterol Education
Program ATP III guidelines.
11
Data collection involved the extraction of data from the patients’
charts using a standardised data tool by trained data clerks. Charts
for patients who attended the clinic from June 2013 to January
2015 were targeted. Those with missing details on key variables
such as cardiovascular risk-factor screening results and ART regimen
were excluded from the data.
Detailed abstraction was then conducted on the remaining
patients’ charts using a data tool that was made up of four
sections, including: (1) anthropometric measures (age, body mass
index, waist circumference and blood pressure), (2) behavioural and
biomedical cardiovascular risk factors (including smoking status,
excessive use of alcohol and non-fasting total cholesterol level), (3)
clinical information (such as on HIV infection and HIV treatment,
ART regimen and duration), and (4) medical history. Data extracted
were entered in a paper data tool then later transferred into an
EpiData software version 3.1 for cleanup in readiness for analysis
using SPSS software.
Statistical analysis
Statistical analysis was performed using SPSS software version 22
(IBM SPSS Statistics, Armonk, NY: IBM Corp). Descriptive statistics
involved calculating the median and interquartile range (IQR)
for continuous data and proportions for categorical variables.
Comparisons of median duration between groups were done
using the Mann–Whitney test with a 5% level of significance.
Associations were assessed using a logistic regression model, and
crude and adjusted odds ratios are reportedwith their corresponding
confidence intervals.
Results
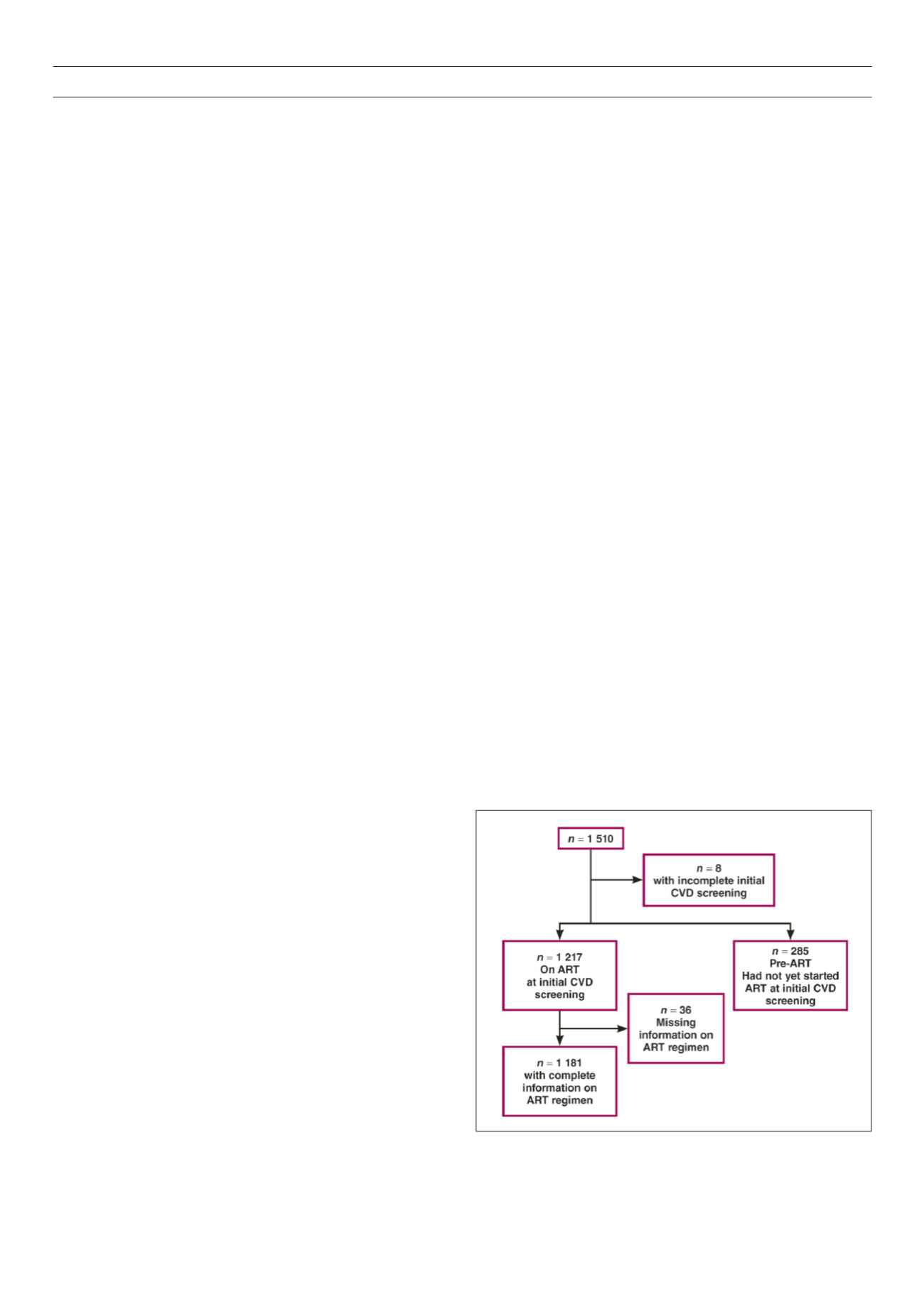
A total of 1 510 subjects was screened, of whom eight were
excluded from analysis because of incomplete data (Fig. 1). Data
collected included demographic variables, risk factors for CVD
and determination of BMI, measurement of blood pressure, and
non-fasting total cholesterol and random blood glucose levels.
Cardiovascular risk score was calculated for those above 40 years
using the WHO (Afri-E) risk-screening chart.
8
Of the subjects screened, 69% (1 036) were women. The
median age was 30 (IQR 31–48) years and median CD4 count was
430 (IQR 308–574) cells/mm
3
; 79% of subjects were on ART with
a documented regimen. Current smokers were 1.9% (29), whereas
0.4% (seven) had known diabetes and 0.3% (four) had had a
previous cardiovascular event (heart attack or stroke).
The median BMI was 21 (IQR 20–23) kg/m
2
with 11% of subjects
underweight, 12% overweight and 2% obese. Waist circumference
was > 100 cm (102 cm) in 1% of men and > 90 cm (88 cm) in 7.5%
of women (Table 1). The median duration on ART was 32.5 (17.4–
50.6) months. Cardiovascular risk-factor distribution stratified by
ART status is shown in Table 2.
Of the 1 502 individuals screened, 40.4% (609/1502) had
pre-hypertension, 10.4% (157/1502) were stage 1 and 2.9%
(43/1502) were stage 2 hypertension. In multivariate analysis,
hypertension was associated with being male [adjusted OR 1.59
(1.26–2.01),
p
= 0.0001], being 40 years or older [adjusted OR
1.78 (1.44–2.21),
p
= 0.0001], and having an increased waist
circumference [adjusted OR 2.56 (1.44–4.55),
p
= 0.0014].
Fig. 1.
Data flow chart for cardiovascular risk screening.



















