
VOLUME 15 NUMBER 1 • JULY 2018
39
SA JOURNAL OF DIABETES & VASCULAR DISEASE
DIABETES GUIDELINES
Because of its low rate of hypoglycaemia and cardiovascular
safety relative to other sulphonylureas, and its proven benefits in
terms of microvascular outcomes, the sulphonylurea of choice is
gliclazide modified release (MR). Glibenclamide should not be used.
Table 2.
Guidelines for management of type 2 diabetes in non-pregnant
adults without metabolic decompensation or cardiovascular disease
Not recommended
Alternative
if HbA
1c
target is
options without attainable with
Preferred
motivation*
other agents
Monotherapy
Metformin XR DPP4i
GLP-1a
Gliclazide MR Insulin
Pioglitazone
SGLT2i
Dual therapy Metformin XR Pioglitazone
GLP-1a
DPP4i
SGLT2i
Insulin
Gliclazide MR
Triple therapy Metformin XR GLP1a
DPP4i
Insulin (basal)
Gliclazide MR SGLT2i
Pioglitazone
Complex
Metformin XR Oral therapy
therapy
+ insulin
+ basal
(pre-mix or basal) insulin + GLP1a
DPP4i: dipeptidyl peptidase-4 inhibitor; SGLT2i: sodiumglucose cotransporter-2
inhibitor; GLP-1a: glucagon-like peptide-1 receptor agonist
*These alternatives do not require motivation to funders as they offer similar
benefits and are selected for the individual circumstances based on clinical
judgement.
From SEMDSA 2017 Guidelines
In patients with symptomatic hyperglycaemia and HbA
1c
> 9%
at diagnosis, initial dual therapy with metformin plus gliclazide
MR should be considered. After optimisation of metformin dose
and lifestyle modification it may be appropriate to discontinue the
sulphonylurea.
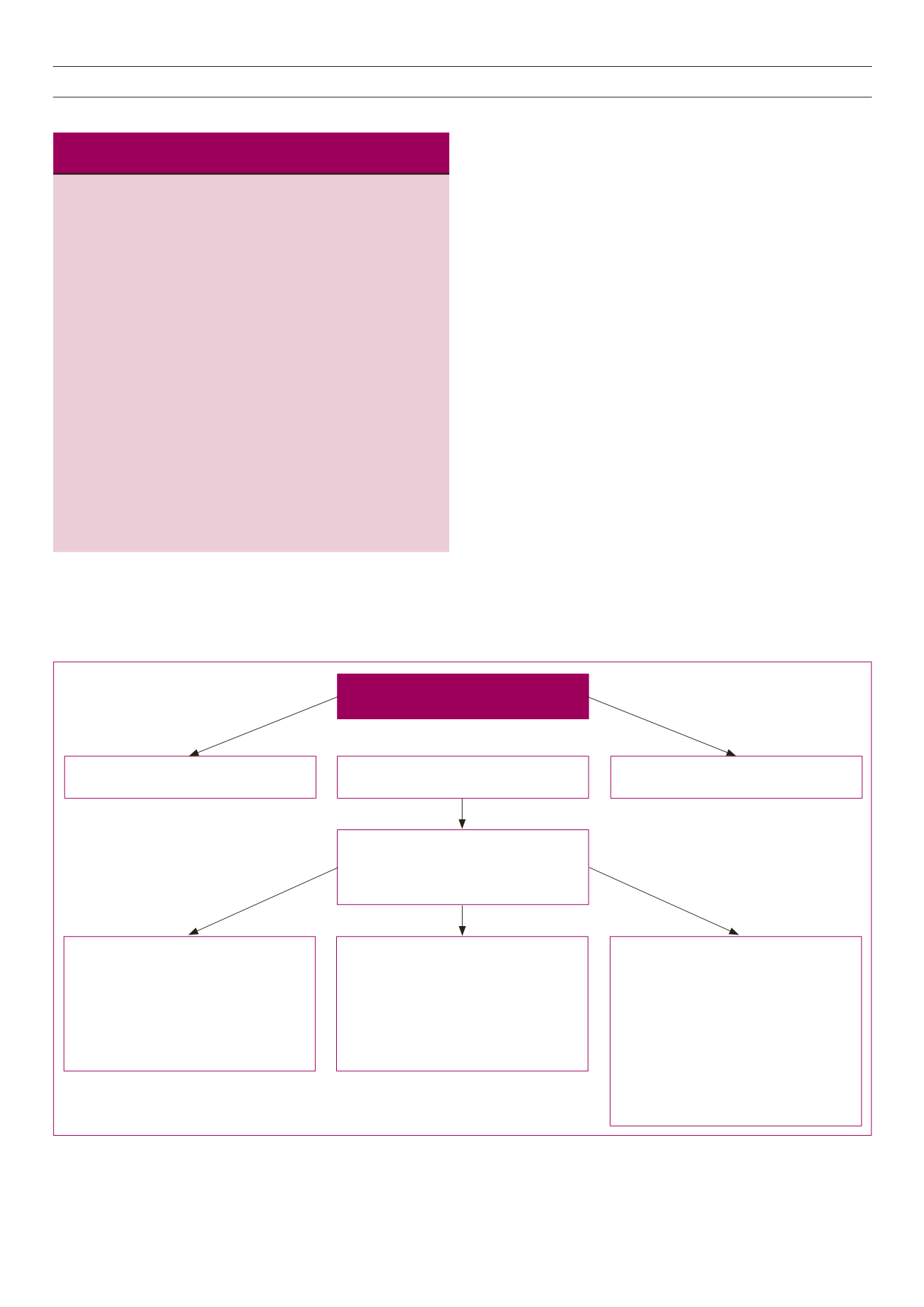
Fig. 1 provides additional advice for triple therapy and initiating
insulin. When selecting additional therapies, consideration should
be given to patient preference, co-morbidities, the individual
properties of each of the pharmacological options and access to
medicines. Expected HbA
1c
reductions are similar when adding a
glucagon-like peptide-1 (GLP-1) receptor agonist or titrated basal
insulin, which are both slightly superior to triple oral therapy. Insulin
initiation must be accompanied by ongoing patient education,
appropriate SMBG, self-titration of insulin doses, frequent review
(initially) and counselling regarding hypoglycaemia. In the absence
of appropriate support for insulin therapy, a third oral agent is
preferred.
A GLP-1 receptor agonist may be preferred to other options
under the following circumstances:
• For overweight and obese patients
• Weight gain or hypoglycaemia has been or is likely to be
problematic with other treatment options (see Hypoglycaemia)
• HbA
1c
is very high
• Patients with established cardiovascular disease (liraglutide
benefit) who are to bemanagedwith specialist-level participation
or responsibility.
Equally, these agents should not be the preferred option:
• In patients in whom weight loss is not desirable
• In patients with chronic gastrointestinal disorders
• In patients with a history of pancreatitis or pancreatic tumours.
Suboptimal glycaemic control with two oral
agents (e.g. metformin + SU)
OPTION 1
Add a 3rd oral agent (e.g. DPP4i)
Ensure there are adequate resources to support
insulin initiation and titration
OPTION 2
Add a GLP-1a
OPTION 3
Add basal insulin (intermediate-acting or
long-acting insulin)
Start with 10 units (0.2 U/kg)
SIMPLE TITRATION
Once weekly average of last two fasting SMBG
level (use pre-prandial SMBG for pre-mix or
bolus insulin):
• If above target: +2 units
• If at target, maintain dose (usual target:
4–7 mmol/l)
• If below target, subtract 2 units
SIMPLE RAPID TITRATION
Once daily titration according to last fasting
SMBG level (use pre-prandial SMBG for pre-mix
or bolus insulin):
• If above target: +1 units
• If at target, maintain dose (usual target:
4–7 mmol/l)
• If below target, subtract 2 units
AGGRESSIVE TITRATION
Once weekly lowest of last three fasting SMBG
readings (use pre-prandial SMBG for pre-mix or
bolus insulin):
> 10.0 mmol/l: +8 units
8.1–10.0 mmol/l: +6 units
7.0–8.0 mmol/l: +4 units
5.6–7.0 mmol/l: +2 units
4.0–5.5 mmol/l: maintain dose
3.1–3.9 mmol/l: –2 units
< 3.1 mmol/l: –4 units
SMBG: self-monitored blood glucose; DPP4i: dipeptidyl peptidase-4 inhibitor; GLP-1a: glucagon-like
peptide-1 receptor agonist.
Fig. 1.
Initiating and titrating basal insulin therapy.



















