94
VOLUME 10 NUMBER 3 • SEPTEMBER 2013
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
18–25 years in Oxford found rates of 8–26%, depending upon the
diagnostic criteria used for PCOS.
15
More recently, an Australian
community study (
n
=728) compared PCOS prevalence using three
different sets of diagnostic criteria: the NIH 1990,
16
Rotterdam
Consensus
17
and the Androgen Excess Society.
18
The women were
predominantly European (94%) with a mean BMI of 25.7 kg/m.
2
Prevalence rates were 17.8% using Rotterdam criteria, and 70%
of these women were previously undiagnosed.
1
The findings
suggest that the actual prevalence of PCOS could be considerably
higher than the frequently quoted 6–10% figure, due to different
diagnostic criteria and under-diagnosis in primary care. This poses
a considerable obstacle if PCOS is to be used as a basis for CMD
screening in clinical practice.
PCOS and obesity
Numbers of women with PCOS appear to be increasing, and this
might indicate increasing prevalence.
19
A link has been reported
between the increasing incidence of obesity, IGT and type 2 diabetes
amongst adolescent girls with PCOS.
20
It is therefore plausible that
the population rise in BMI might be increasing the prevalence of
PCOS, since obesity is known to increase hyperandrogenism
21
and
menstrual irregularity.
21,22
Of potential significance is the associated
increase in insulin resistance observed in these women.
23–25
Several
studies suggest that PCOS may be a specific manifestation of
insulin resistance,
6
and that insulin resistance is present in the
majority.
26
Thus, it would not be surprising if the global rise in
obesity is increasing the prevalence of PCOS and associated
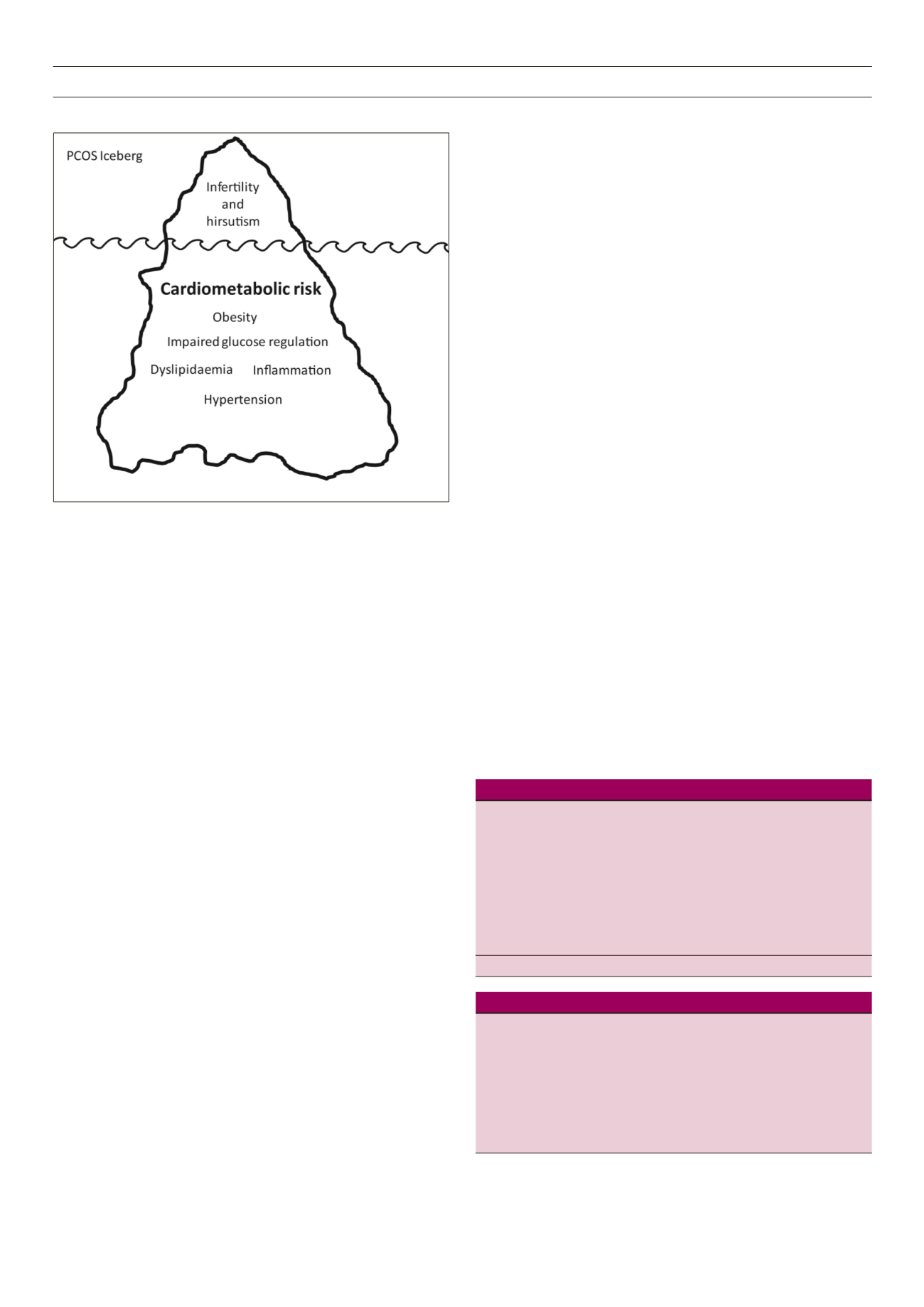
CMD linked to insulin resistance. However, it is likely that women
currently diagnosed with PCOS are the tip of a very large iceberg
with the majority of PCOS and the co-existing CMD unrecognised
and untreated (Figure 1).
Current guidance on screening for type 2 diabetes in women
with PCOS
Both DUK and the ADA recommend that women diagnosed with
PCOS are screened for type 2 diabetes, although US guidance
suggests screening if women have a BMI ≥ 25 kg/m,
2
whereas DUK
suggest screening if BMI ≥ 30 kg/m
2.2,3
Currently, neither DUK nor
ADA provide guidance on the optimal screening test for type 2
diabetes inwomenwith PCOS, the screening interval or interventions
to reduce women’s health risks. There is a need for evidence-based
guidelines on screening, diagnosis and interventions to reduce CMD
specifically in women with PCOS. Current screening guidelines are
based upon evidence extrapolated from studies of older, mixed sex
populations whereas PCOS is a condition of women in reproductive
years and therefore quite clearly distinct.
Controversies in screening and diagnosis of PCOS
Several different sets of diagnostic criteria are used to define PCOS,
and the lack of consensus causes confusion and undoubtedly
contributes to under-diagnosis and difficulties in determining whom
to screen for CMD. The three main sets of criteria are as follows.
The NIH 1990 criteria
There was no agreed definition of PCOS until the NIH 1990
conference sought consensus expert opinion through questionnaires
and debate, producing the first PCOS diagnostic criteria.
16
Although
the term ‘PCOS’ itself implies that PCO should be an essential
feature for diagnosis, this was controversial and ‘polycystic ovaries’
were not included in the final NIH criteria (Table 1).
Using NIH classification, women with hyperandrogenism and
regular menstrual cycles, and women with PCO, irregular menstrual
cycle but no hyperandrogenism, would not be diagnosed as
having PCOS. There was concern that these criteria could result
in false negative diagnoses, missing those with regular cycles,
hyperandrogenism and PCO. The debate over the use of ‘polycystic
ovaries’ as a diagnostic criterion continued until 2003 when a
conference in Rotterdam proposed different criteria.
17
The Rotterdam (ESHRE) 2003 criteria.
In 2003 the ESHRE/ASRM
developed what are now known as the ‘Rotterdam criteria’. These
added two new phenotypes by including the feature of PCO in the
diagnostic criteria (Table 2):
Table 1.
The NIH 1990 criteria for the diagnosis of PCOS.
A diagnosis of PCOS may be made if there is both:
i. Presence of hyperandrogenism with either clinical signs (hirsutism, acne,
or male pattern balding) or biochemical signs of hyperandrogenemia
(high serum androgen concentrations) and
ii. Presence of chronic menstrual irregularity due to oligomenorrhoea/
amenorrohoea
after
iii. Excluding other known disorders such as Cushing’s syndrome,
Congenital Adrenal Hyperplasia, androgen-secreting tumours, and hyper-
prolactinaemia
PCOS: polycystic ovary syndrome.
Table 2.
The Rotterdam 2003 criteria for the diagnosis of PCOS.
A diagnosis of PCOS may be made if any two of the following features are
present:
i. The presence of menstrual irregularities – oligomenorrhoea and/or
annovulation
ii. The presence of clinical and/or biochemical signs of hyperandrogenism
iii. The presence of polycystic ovaries
after
iv. Excluding other potential causes of menstrual irregularity or hyper-
androgenism
IcebergTomlinson and Pinkney ©2013
Figure 1.
Polycystic ovary syndrome (PCOS) iceberg.


