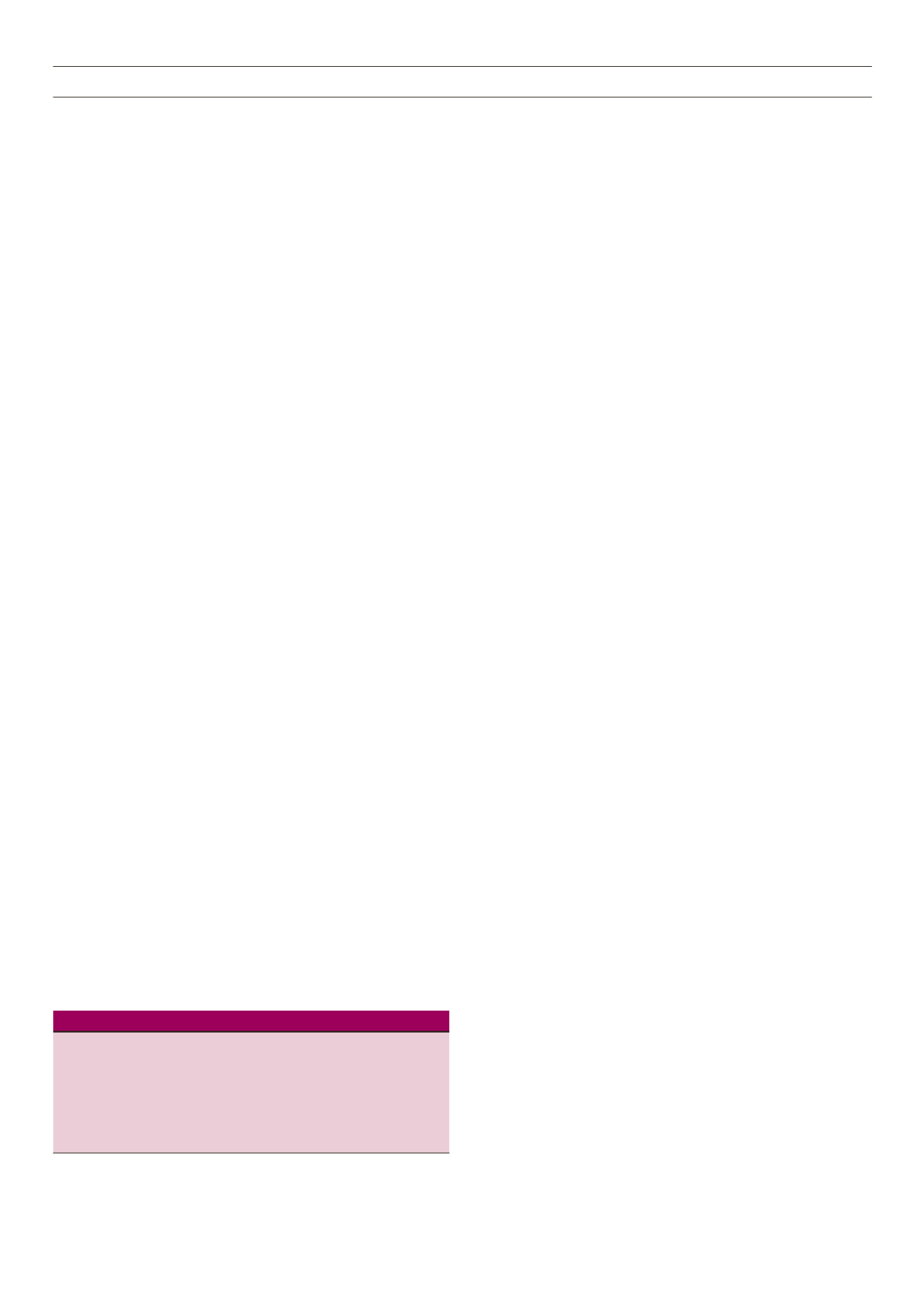
SA JOURNAL OF DIABETES & VASCULAR DISEASE
REVIEW
VOLUME 10 NUMBER 3 • SEPTEMBER 2013
95
• PCO, androgen excess (but normal ovulation and menstrual
cycles),
• PCO, abnormal ovulation and menstrual cycles (without
evidence of hyperandrogenism).
27
The Rotterdam criteria increase the number of women meeting
criteria for PCOS, although ovarian ultrasound would be required if
these criteria were to be fully adopted in primary care. Thus, despite
Rotterdam criteria being widely recommended in the UK, in the
absence of ultrasound data many women can only be diagnosed
using the NIH features of oligomenorrhoea and hyperandrogenism.
This would lead to failure to diagnose women with PCO and either
hyperandrogenism or oligomenorrhoea.
It remains debatable whether NIH or Rotterdam are the
appropriate diagnostic criteria for PCOS as each definition gives two
overlapping, yet phenotypically different sets of women. Although
Rotterdam criteria include a wider range of phenotypes, in terms
of CMD and insulin resistance, NIH phenotypes (hyperandrogenism
and chronic anovulation) probably carry greater CMD risk
28
than
those who would only be diagnosed through Rotterdam criteria.
Clearly, there remain inherent problems with both criteria and there
is no accepted ‘gold standard’. Supporters of Rotterdam argue
that NIH fails to identify some PCOS, whereas others argue that
Rotterdam has broadened the diagnostic criteria too far. This lack
of consensus has substantial implications for diabetes screening
and potential CMD risk reduction in women with PCOS.
The AES recommendations
This diagnostic controversy prompted the AES to
make its own recommendations for diagnostic criteria (Table 3).
The AES criteria.
AES allow the use of ultrasound, but unlike
Rotterdam, hyperandrogenism is regarded as essential for diagnosis.
However, a minority still considered that some women may have
PCOS in the absence of hyperandrogenism, and so the definition is
likely to continue to evolve as more evidence becomes available.
27
What is apparent is that until there is a universally accepted
definition of PCOS, it will be difficult to fully resolve which women
to screen for type 2 diabetes and who might benefit from measures
to reduce CMD risk.
Difficulties using clinical features in the diagnosis
of PCOS
PCO
Ultrasound examinations demonstrate that PCO are present in
32–33% of young women, are not necessarily related to other
symptoms or metabolic dysfunction
15,29
and are especially unreliable
for diagnosing PCOS in adolescents.30 A diagnosis of PCOS using
PCO as a criterion, and without evidence of hyperandrogenism, is
therefore controversial. Despite inclusion of PCO in the Rotterdam
criteria, requests for ultrasounds are often not accepted by imaging
departments in the UK for diagnosis of PCOS. This leaves clinicians
with no alternative than to use NIH criteria, unless previous scans
are available or if secondary care specialists make requests. Where
ultrasound information is available, clinicians should be wary of
relying on interpretive comments of non-expert reporters such as
‘consistent with PCOS’. Clinical reports often lack standardisation
in the interpretation with only poor to moderate levels of inter-
observer agreement.
31
Thus, there should be reliable evidence of the
size and number of ovarian cysts before an ovarian scan report can
be considered reliable for diagnosis. In addition, ovarian ultrasounds
are often performed trans-abdominally, rather than transvaginally,
and can be of inferior quality.
32,33
Transvaginal scans (although more
invasive than trans-abdominal) have been found to be superior to
transabdominal for identifying ovarian cysts in obese women.
33
They are therefore the preferred modality for ovarian imaging in
women with suspected PCOS. In summary, limited access to high
quality ultrasound examination in primary care is one of the factors
constraining the identification and classification of women with
PCOS, and therefore has implications for identifying the population
to be screened for type 2 diabetes and CMD risk.
Hyperandrogenism: biochemical
There are also difficulties in using hyperandrogenism as a guide
to potential PCOS. Further challenges are found in the assessment
of hyperandrogenism (CH and BH). BH can be assessed in several
different ways and there is no agreed standard biochemical test, or
reference range. It is widely considered that the most informative
index of BH is a combined measurement of a raised total s-T and
a decrease in its principal binding protein – SHBG, although these
measurements in isolation are not always indicative of PCOS. A
low SHBG is a marker of insulin resistance, and can be of use for
identifying insulin-resistant individuals
34
which may help to identify
those with greatest CMD risk. However, reduced levels of SHBG are
certainly not specific to PCOS.
Total s-T measurements do not provide information on
testosterone bioavailability. Some laboratories offer measurements
of f-T, although this is relatively expensive and not widely available.
The FAI is an alternative index of hyperandrogenism, calculated
by dividing the t-T measurement by the SHBG and multiplying by
100 to obtain an estimate of the f-T level. As hyperandrogenism is
often difficult to fully assess in women who are taking oestrogen
containing contraception, guidelines recommend measuring s-T
prior to initiating COC or after a 3-month washout.
30
The role of SHBG in the diagnosis of hyperandrogenism is also
not straightforward. Serum SHBG levels are inversely associated with
insulin levels.
35
Thus, low SHBG levels and consequently elevated FAI
areoften found in insulin-resistantwomen, including thosewithPCOS.
The pathogenesis of hyperandrogenism is often considered likely to
result from obesity-induced hyperinsulinaemia and suppression of
SHBG production by the liver. The reverse may then be seen when
weight loss triggers a reduction in insulin, elevation of SHBG and
drop in the FAI. These effects have been noted when women who
have PCOS lose significant amounts of weight after bariatric surgery,
indicating that obesity exacerbates hyperandrogenism, increased
fasting insulin and reducing SHBG.
36,37
Hyperandrogenism: clinical
The diagnosis of CH is also complicated by subjectivity and wide inter-
individual variation. Hirsutism is a good marker for hyperandrogenism
Table 3.
The Androgen Excess Society criteria for the diagnosis of PCOS.
A diagnosis of PCOS may be made if the following features are present:
i. The presence of hyperandrogenism (clinical and/or biochemical) and
ii. The presence of ovarian dysfunction (oligo-anovulation and/or polycystic
ovaries)
after:
iii. Excluding other causes