SA JOURNAL OF DIABETES & VASCULAR DISEASE
REVIEW
VOLUME 7 NUMBER 4 • NOVEMBER 2010
145
Limitations
Limitations of these studies include being restricted to special
populations like women,
16
women with osteoporosis
18
or nurses.
17
Another common limitation is the ascertainment of diabetes by self-
report
16,17,20
or even mailed self-report questionnaire.
19
The cognitive
assessment was sometimes performed by telephone,
17,19
which may
be less reliable than examinations by qualified or trained assessors.
One study introduced two additional cognitive tests during
follow-up (Digit Symbol Test and Trails B test) but the only test
used at baseline and follow-up (modified MMSe) did not generate
statistically significant data.
16
The utilisation of confounders varied
among the studies ranging from only a few confounders (age,
education, race and depression)
18
to a broad spectrum of covariates
(age, education and hormone use in women, baseline score, BMI,
hypertension, hypercholesterolaemia, depression, smoking, alcohol
and physical activity).
19
Summary
Though there are many hints of a causal association between
diabetes and the development of cognitive decline, definitive proof
of a protective effect of antidiabetic treatment by controlled or
even randomised placebo-controlled studies is still required.
Hyperlipidaemia
Pathological and experimental data suggest that cholesterol
may play a role in the pathogenesis of cognitive impairment and
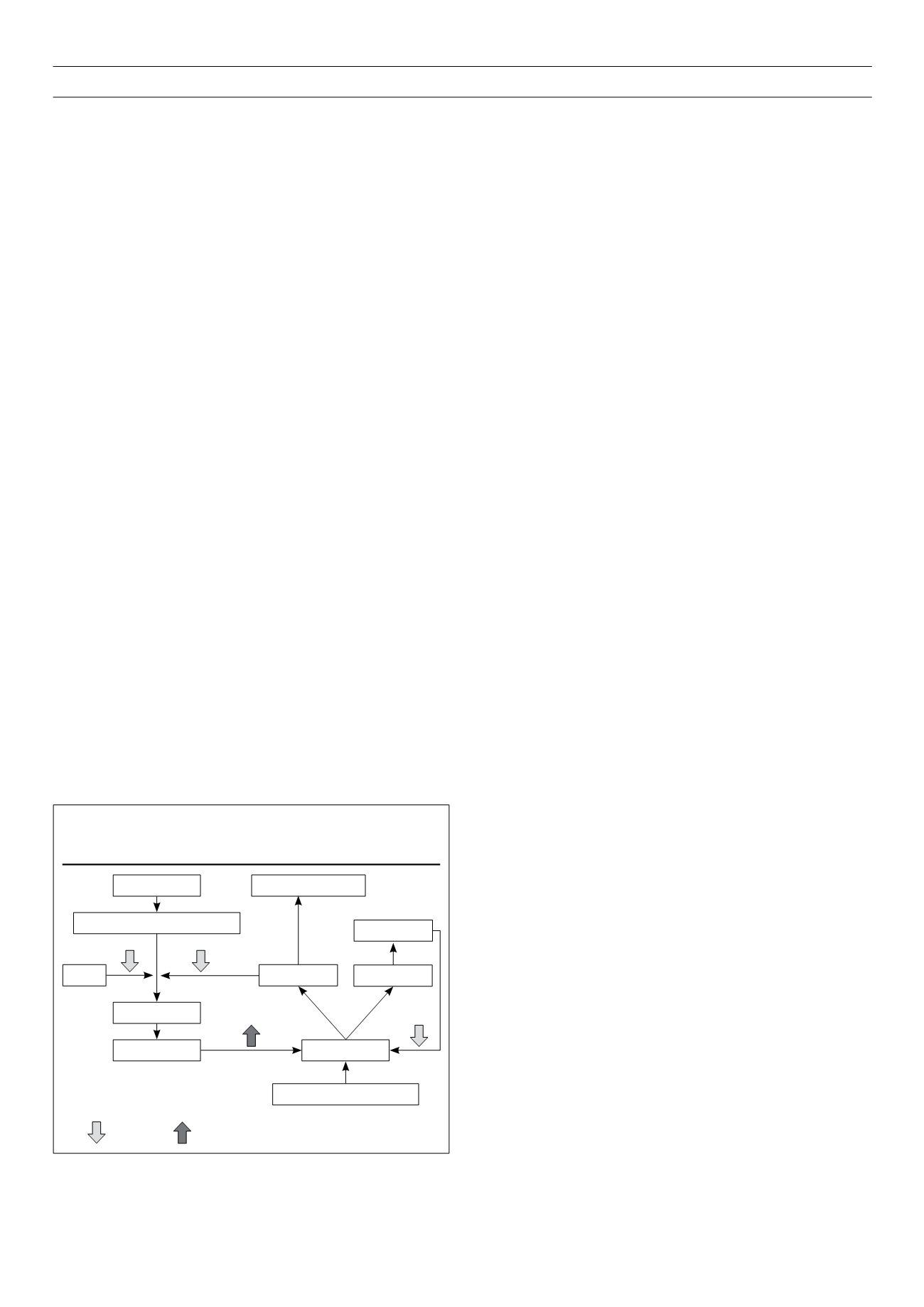
dementia (Fig. 1).
23
A relationship between amyloid deposition and
serum hypercholesterolaemia in the human brain was detected
in autopsy cases of patients older than 40 years.
24
An association
between severe circle of Willis atherosclerosis and sporadic
dementia was found among 54 autopsy cases.
25
Studies on hyperlipidaemia and cognitve decline
The finnish CAIDe study evaluated the impact of midlife serum
cholesterol levels on the subsequent development of mild cognitive
impairment among 1 449 participants. After an average follow-up
of 21 years, a midlife elevated serum cholesterol level (
≥
6.5
mmol/l) was a significant risk factor for MCI (oR 1.9; 95% CI 1.2–
3.0) after adjustment for age and BMI.
26
In a retrospective cohort
study of 8 845 participants, aged 40–44 years, high cholesterol
was associated with a 40% (HR 1.42; 95% CI 1.22–1.66) increase
in risk of dementia after 30 years.
27
The french Three-City-Study, a
population-based cohort of 9 294 subjects, reported an increased
risk for subjects with hyperlipidaemia (OR 1.43; 95% CI 1.03–1.99)
independent of all major potential confounders.
28
In contrast to these trials, studies with participants of older age
couldnot confirmthis result of elevated cholesterol beinga risk factor.
In the Italian longitudinal Study on Aging with 2 963 participants
(age 65–84 years), serum total cholesterol had a borderline (non-
significant) trend for a protective effect.
29
A population-based
cohort study with 2 356 participants, aged 65 and older, found no
association between cholesterol and subsequent risk of dementia
after adjustment for several cofounders (age, sex, education,
baseline cognition, vascular comorbidities, BMI and lipid-lowering
agent use).
30
In a cross-sectional and prospective community-based
cohort study including 4 316 Medicare recipients, 65 years and
older, only a weak relationship between cholesterol levels and the
risk of vascular dementia was observed.
31
Several reasons could explain the paradoxical situation
that hypercholesterolaemia is apparently a risk factor in
midlife but becomes a more protective factor in older age.
Hypercholesterolaemia in the elderly may reflect a good dietary
status and overall health, whereas low levels of cholesterol could
be the result of poor nutrition caused by cognitive decline or other
medical problems.
32
Studies with statins
The majority of observational and prospective studies suggested
an association between statin use and cognitive decline, especially
Alzheimer’s dementia.
28,33,34
A population-based cohort study
comprising 1 674 older Mexican Americans with a five-year
follow-up found, after adjustment for several risk factors, that
statin users were about half as likely as non-statin users to develop
cognitive decline (HR 0.52; 95% CI 0.34– 0.80).
35
In the prospective
population-based Rotterdam Study with 6 992 participants and a
mean follow-up of nine years, statin use was associated with a
decreased risk of Alzheimer dementia (HR 0.57; 95%CI 0.37–0.90),
compared with no use of cholesterol-lowering drugs. There was no
difference between lipophilic (simvastatin, atorvastatin, cerivastatin)
or hydrophilic statins (pravastatin, fluvastatin, rosuvastatin), but
non-statin cholesterol-lowering drug use (fibrates, nicotinic acid,
etc.) was not effective.
36
On the other hand, two large placebo-controlled trials found no
positive association between statin therapy and cognitive decline.
The HPS reported no protective effect of simvastatin on cognitive
decline after five years among 20 536 high-risk vascular participants
aged between 40 and 80 years.
37
In the PRoSPeR trial, which
included 5 804 elderly high-risk vascular participants (aged 70–82
years), pravastatin had no significant effect on cognitive function.
38
A recent Cochrane review based on these two randomised trials
concluded that statins given in late life to individuals at risk of
vascular disease have no effect in preventing dementia.
39
Detailed
analyses of the latter study designs might explain the negative
results despite the large number of participants. Both studies
were not primarily designed to assess cognitive function. neither
included a baseline measurement of cognitive function, which
makes an accurate evaluation of any statin effect difficult.
40
The
Figure 1.
Simplified model of metabolic pathway of cholesterol and
amyloid
b
23
Key:
= inhibition,
= upregulation
Acetyle-CoA
3-HMG-3-Methyl-glutaryl-CoA
Statins
Mevalonat
Cholesterol
Tau phosphorylation
Sphingolipids
Amyloid
b
40 Amyloid
b
42
y-Secretase
Amyloid precursor protein


