
60
VOLUME 17 NUMBER 2 • NOVEMBER 2020
Report
SA JOURNAL OF DIABETES & VASCULAR DISEASE
sitagliptin, vildagliptin) have a low risk of hypoglycaemia and
have been associated with improved glycaemic control, insulin
secretion and
β
-cell function, they are considered to be weight
neutral or associated with minimal changes in weight. They are
very safe drugs, seldom give rise to side effects and are ideal for
use in the elderly or those patients at high risk of hypoglycaemia.
DPP-4 inhibitors are generally neutral from a cardiovascular point
of view, but some should not be used in patients with heart
failure.
Thiazolidinediones
The thiazolidinediones are associated with weight gain, making
pioglitazone less favourable for patients with diabesity. However,
pioglitazone improves NAFLD, although its use is associated with
increased risk of heart failure, urinary bladder cancer, secondary
osteoporosis and fractures.
15
Thiazolidinediones can be useful in
patients with extreme insulin resistance and have demonstrated
good stroke prevention data in the IRIS study.
Insulin
Because it is an anabolic hormone, insulin causes weight
gain through inhibition of protein catabolism, stimulation of
lipogenesis, slowing of basal metabolism and increasing the
accumulation of fat. Increase in body weight and fat mass is
strongly associated with the intensity of the insulin regimen, as
well as worsening of diabesity. Insulin in high doses, as often used
in obese diabetics, can cause massive weight gain. Yet insulin
and sulphonylureas (gliclazide, glimepiride, glipizide, glyburide)
are frequently used early in the management of T2DM. Gliclazide
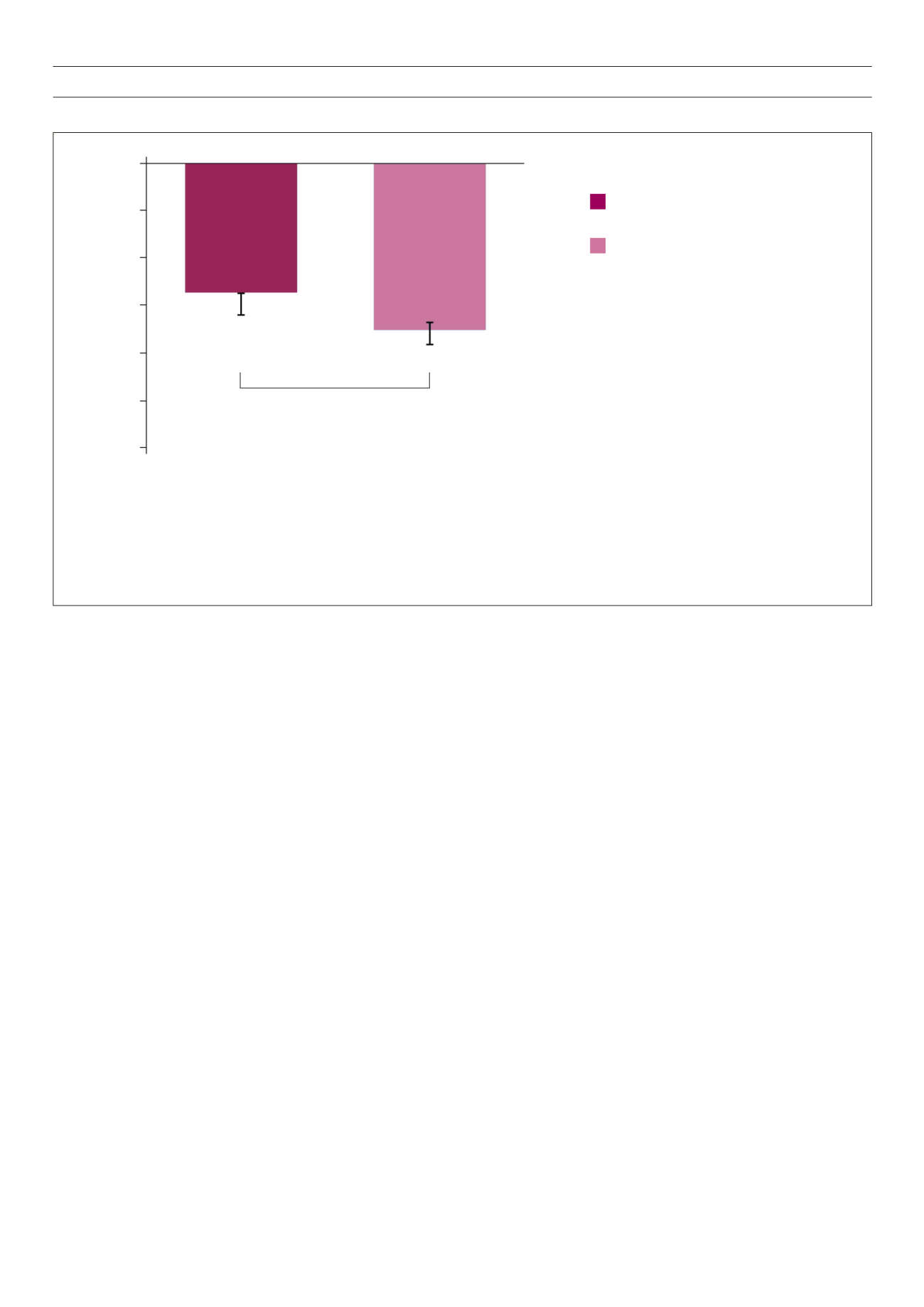
Fig. 5.
AWARD-6: Liraglutide is superior to dulaglutide for weight loss.
is the only sulphonylurea recommended by the Society for
Endocrinology, Metabolism and Diabetes of South Africa and is
associated with minimal weight gain.
For patients with obesity and T2DM requiring insulin therapy,
the Endocrine Society Clinical Practice Guideline recommends
concomitantly prescribing at least one weight-loss promoting
medication (e.g. metformin, GLP-1 RAs or pramlintide) to mitigate
associated weight gain from insulin use.
5,15
With all the new agents available, especially the weight-friendly
GLP-1 RAs and SGLT-2 inhibitors, these drugs should be optimised
before insulin is considered. Insulin should only be used if no other
options are left.
4
Clinical focus with Dr Lombard
Multidrug treatment for glycaemic control: keeping weight
top of mind
Drugs with prognostic (survival) benefits should be used first. It
is interesting to note that the only classes that have prognostic
benefits are also those medications that contribute to weight loss
– metformin, SGLT-2 inhibitors and GLP-1 RAs. Variations in drug
efficacy within classes imply that the right choices need to be made
for each patient – ‘the art of medicine’. Dr Lombard observes how
very unfortunate it is that medical funders usually do not see the
point of individualised management, and these drugs are often
poorly reimbursed. They would rather pay for the complications
than pay to prevent them. Fixed combinations are entering the
market and will offer many more excellent choices, with very robust
published data to support their use.
Most patients with T2DM require more than one antidiabetic
Dulaglutide 1.5 mg
(
n
= 299; baseline weight: 93.8 kg)
Liraglutide 1.8 mg
(
n
= 300; baseline weight: 94.4 kg)
–3.61
–2.90
p
< 0.05
Mean weight change from baseline
(kg, mean ± SE)
0
–2
–3
–4
–5
–6
–1
• 26-week, active-controlled, phase 3 non-inferiority study (AWARD-6)
• Treatment was added to background therapy with metformin
• Primary endpoint was met: non-inferiority of dulaglutide 1.5 mg vs liraglutide 1.8 mg in HbA
1c
reduction from baseline to 26 weeks (–1.42 vs –1.36%,
p
< 0.001 for non-inferiority)
• Dulaglutide is not indicated for weight loss. In AWARD-6, weight change was a secondary endpoint.
In AWARD studies 1–5, mean weight change was –3.2 to –0.9 kg for dulaglutide 1.5 mg
• All
n
values refer to ITT population



















