24
VOLUME 11 NUMBER 1 • MARCH 2014
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
Evidence from a cohort of patients undergoing autologous stem
cell transplantation observed that hyperglycaemia was associated
with failed haematopoietic stem cells (HSC) mobilisation from the
osteoblastic niche into the peripheral circulation.
11
This observation
was corroborated by data from a prospective trial involving 24
patientswithdiabetes,which reported reduced stemcellmobilisation
in response to granulocyte colony stimulating factor (G-CSF) relative
to non-diabetic patients.
12
Putative mechanisms underlying this
include failure of CD26/DPP-4 upregulation on CD34+ stem cells
in diabetic patients, which is required for G-CSF-induced stem cell
mobilisation.
12
These findings suggest the bone marrow of diabetic
patients has an impaired capacity to release HSCs following
stimulation with G-CSF. Furthermore, in a rodent model of diabetes
an increased density of HSCs within the bone marrow following
G-CSF stimulation was observed with no commensurate increase in
circulating HSC number. Interestingly, the penchant of HSCs for the
bone marrow was diminished following transplantation into non-
diabetic mice.
13
This latter observation suggests diabetes impairs
HSC mobilisation by altering the bone marrow micro-environment
and does not intrinsically affect HSCs
per se
.
The vascular sinusoids of the bone marrow, which is the
interface for cell exchanges with the peripheral circulation, have
been identified as a target for diabetes-induced bone marrow
microangiopathy. In support of this, evidence from a rodent model
of diabetes reported that cultured Sca-1POS c-KitPOS stem cells
from the bone marrow showed higher levels of oxidative stress,
DNA damage, precocious senescence, apoptosis and reduced
vasculogenic properties. In the rodent models diabetes was
associated with microvascular rarefaction and critical bone marrow
ischaemia and hypoxia with associated HSC depletion, attenuated
transendothelial stem cell mobilisation and fatty degeneration.
14
Furthermore, down-regulation of the polycomb gene Bmi-1, which
normally represses genes that induce cellular senescence and
apoptosis and maintains stem cell pluripotency, were observed in
rodent models of diabetes.
15
Recent evidence using femoral bone samples from patients with
type 2 diabetes and non-diabetic controls obtained during hip
replacement surgery observed a reduction in CD34+ stem cells with
increased apoptosis in diabetic patients. This was associated with
increased expression and nuclear localisation of the pro-apoptotic
factor FOXO3a and its targets, p21 and p27. In HSCs with DNA
damage, FOXO3a induces cell cycle arrest through transcriptional
regulation of the cyclin-dependent kinase inhibitor p27Kip1 and
Bcl-2 family member Bim. Cell cycle analysis showed that CD34+
stem cells from the bone marrow of diabetic patients were
arrested in G1 phase – the typical restriction checkpoint where
HSCs are cycle-arrested to prevent accumulation of DNA damage.
Furthermore, levels of microRNA-155 (miRNA-155), which holds
HSCs at an early stem and progenitor state through inhibition of
differentiation-associated molecules and regulates stem cell survival
through inhibition of FOXO3a, was significantly reduced in CD34+
stem cells from diabetic patients. Exposure of healthy CD34+ stem
cells to hyperglycaemia was associated with down-regulation
of miRNA-155. Forced expression of miRNA-155 reversed the
repressive transcriptional effects associated with hyperglycaemia-
induced upregulation of FOXO3a, p21 and p27
kip1
, which suggests
miRNA-155 may be a potential therapeutic candidate agent to
ameliorate diabetes-induced bone marrow microangiopathy. It
was also observed that diabetes had a selective deleterious effect
on haematopoietic cell lineages within the bone marrow with
preservation of both B- and T-lymphocyte and natural killer cell
numbers.
16
Evidence on the time course of stem cell diminution in diabetes
suggests that CD34+ stem cell counts are reduced in both the
bone marrow and circulation at time of diagnosis, partially recover
during the subsequent two decades and fall again thereafter with
exhaustion of the bone marrow stem cell reserve in the long-
term.
17
Stem cells for the treatment of diabetes
The realisation that islet cell transplantation can potentially cure
diabetes by replenishing deficient pancreatic islet cells stemmed
an interest in regenerative approaches to diabetes care (Fig. 1).
The relative scarcity of donations for pancreas or islet allograft
transplantation has prompted the search for alternative sources
for islet cell replacement therapy. There is emerging evidence
supporting the efficacy of ES, adult and iPS cells in this regard.
The mainstay of evidence supports their utility in type 1 diabetes.
However, patients with type 2 diabetes who require exogenous
insulin may also benefit from stem cell therapy, considering the
occurrence of progressively worsening
β
-cell failure.
Embryonic stem cells
Human ES cells were first studied as a model system for lineage-
specific differentiation into pancreatic islet cells over a decade ago.
Studies using human ES cells in adherent and suspension culture
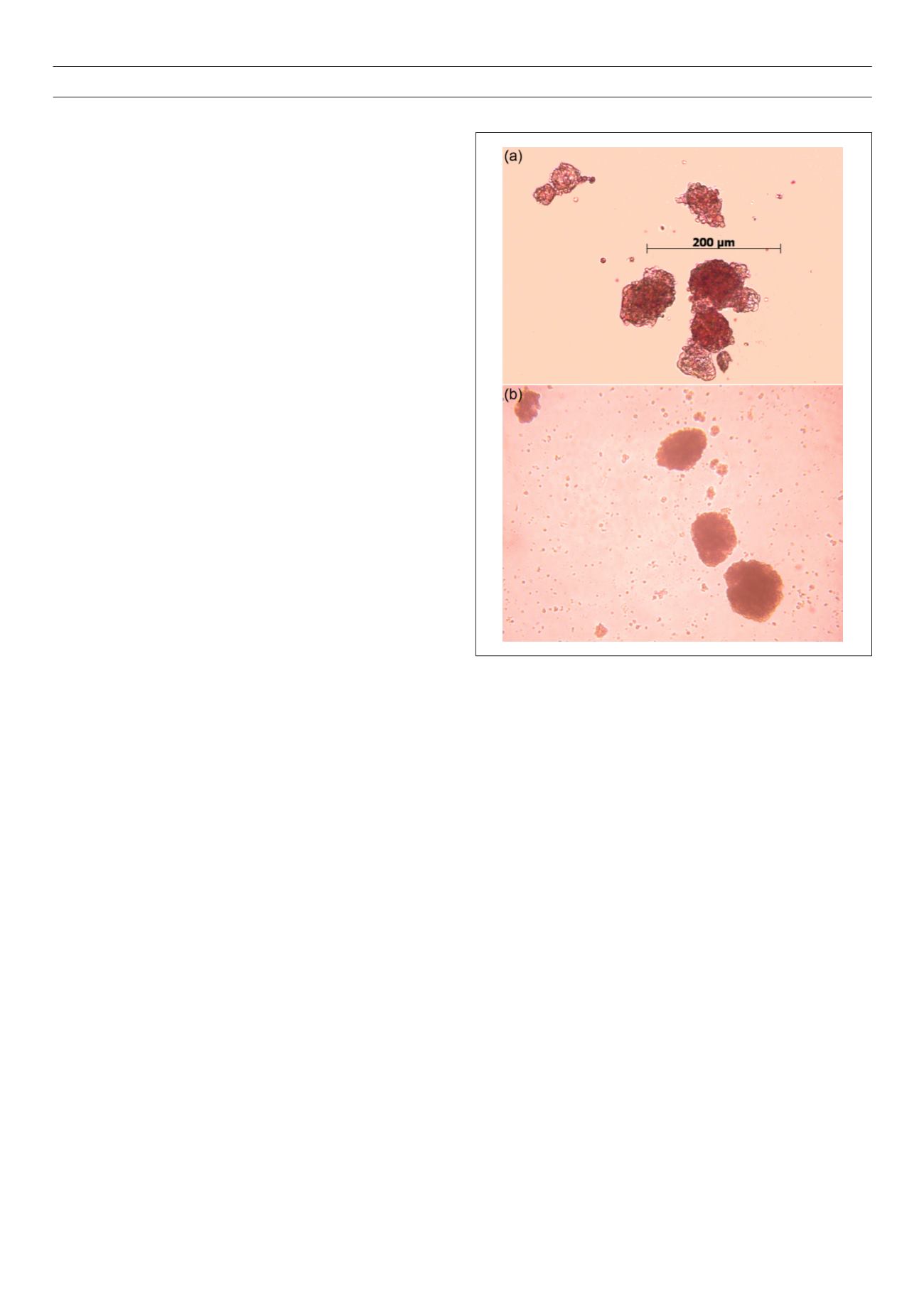
Figure 1.
Isolated human islets.
The authors thank the Islet Research Laboratory in Worcester, UK for supplying these photos.


