28
VOLUME 11 NUMBER 1 • MARCH 2014
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
incretins and their interactions in glucose/energy homeostasis and
gut biology are still unclear.
Incretin-based therapies are relatively new additions to the
management of type 2 diabetes (Fig. 2). They are either inhibitors
of the DPP-4 enzyme (sitagliptin, vildagliptin, saxagliptin and
linagliptin), which prolong the effect of endogenous GLP-1, or
analogues of GLP-1 (exenatide, liraglutide and lixisenatide) that are
resistant to the action of the DPP-4 enzyme. The inhibitors of DPP-4
generally reduce the enzyme’s activity in serum by 80%, which
causes a doubling of postprandial levels of biologically active native
GLP-1.
12,13
Compared with the sustained pharmacological increases
in the serum levels of GLP-1 analogues, DPP-4 inhibitors produce a
relatively modest and transient rise in postprandial GLP-1.
14
Hence,
the GLP-1 analogues are more efficacious in reducing body weight
and achieving glycaemic control.
As the insulinotropic effect of GLP-1 occurs only when plasma
glucose levels are raised, incretin-based therapies have a lower
propensity to cause hypoglycaemia. Incretin-based therapies have
rapidly become an established component of our armamentarium
for the treatment of type 2 diabetes. Their main advantages are
the weight-losing/neutral properties and the relatively low risk of
hypoglycaemia.
The receptor for GLP-1 is expressed in a number of human tissues,
including the pancreas, intestine, lung, kidney, breast, brain, heart
and endothelium; and also in various endocrine tumours, especially
pheochromocytomas.
16
However, the receptor is thought to be
absent or sparsely expressed in human liver, spleen, lymph nodes
or adrenal gland.
16
Therefore, it is likely that the clinical effect of
endogenous GLP-1 and incretin-based drugs is not just confined to
glycaemic control mediated through insulin release, but could also
include actions on a diverse array of tissues that are implicated in
many physiological and pathological processes.
In this brief review article, we outline the main physiological
effects of GLP-1 (Table 1). We also hypothesise and explore novel
pharmacological effects of incretin-based therapies, based on the
known distribution of GLP-1 receptors, and provide a focus for
future research in this field.
Effects of GLP-1on blood pressure
In a series of large, randomised clinical trials termed LEAD
(Liraglutide Effect and Action in Diabetes),
17
liraglutide produced
a modest but significant reduction (between 2 and 6 mmHg) in
systolic blood pressure, without any significant change in diastolic
blood pressure.
18–20
Subjects on liraglutide therapy also experienced
a significant reduction in their body weight, which might have
accounted for at least some of the change in blood pressure;
however, in one of the studies, the improvement in blood pressure
occurred before any substantial weight loss occurred, suggesting a
weight-independent mechanism by which GLP-1 influences blood
pressure.
21
A trend towards a significant reduction in systolic blood
pressure was also noted during exenatide treatment.
22
Experiments using rat models suggest that incretin-based
therapies lower blood pressure by modulating central and
peripheral neural pathways.
23
GLP-1 also seems to have diuretic and
natriuretic properties that may explain the anti-hypertensive effect.
24
Recent evidence from experiments on mice hearts demonstrates
the secretion of ANP following activation of the GLP-1 receptor in
the atrium.
25
Blood pressure may be regulated via the gut–heart
axis, in which GLP-1 and atrial natriuretic peptide (ANP) play a role.
Preclinical studies show favourable effects on endothelial function
in animals, for example: stimulation of nitric oxide production
and reduced production of adhesion molecules.
26,27
A recent meta-
analysis shows an increase in the heart rate of 1.86 (95% CI 0.85–
2.87) in patients treated with GLP-1 analogues. This increase was
more pronounced for long-acting drugs such as liraglutide and a
once-weekly preparation of exenatide.
28
The anti-hypertensive effect is confined to systolic blood
pressure and is variable in clinical trials. Therefore, GLP-1 analogues
should be currently regarded as a treatment for diabetes only, and
additional anti-hypertensives should be considered in patients with
raised blood pressure.
Effects of GLP-1 on the heart
In one clinical study, continuous GLP-1 infusion was given for 72
hours to 10 patients with an ejection fraction < 40%, following
an acute myocardial infarction and successful primary angioplasty.
When compared with 11 subjects treated with placebo, the GLP-1
treated patients had a significant improvement in left ventricular
ejection fraction, from 29 ± 2 to 39 ± 2% (
p
< 0.01). Interestingly,
this was noted in patients both with and without diabetes, and
independent of infarct location.
29
In a recent study, 172 patients
with myocardial infarction were randomised to receive exenatide
or placebo infusion for 6 hours, commencing 15 minutes prior to
primary angioplasty. The infarct size in relation to the area at risk
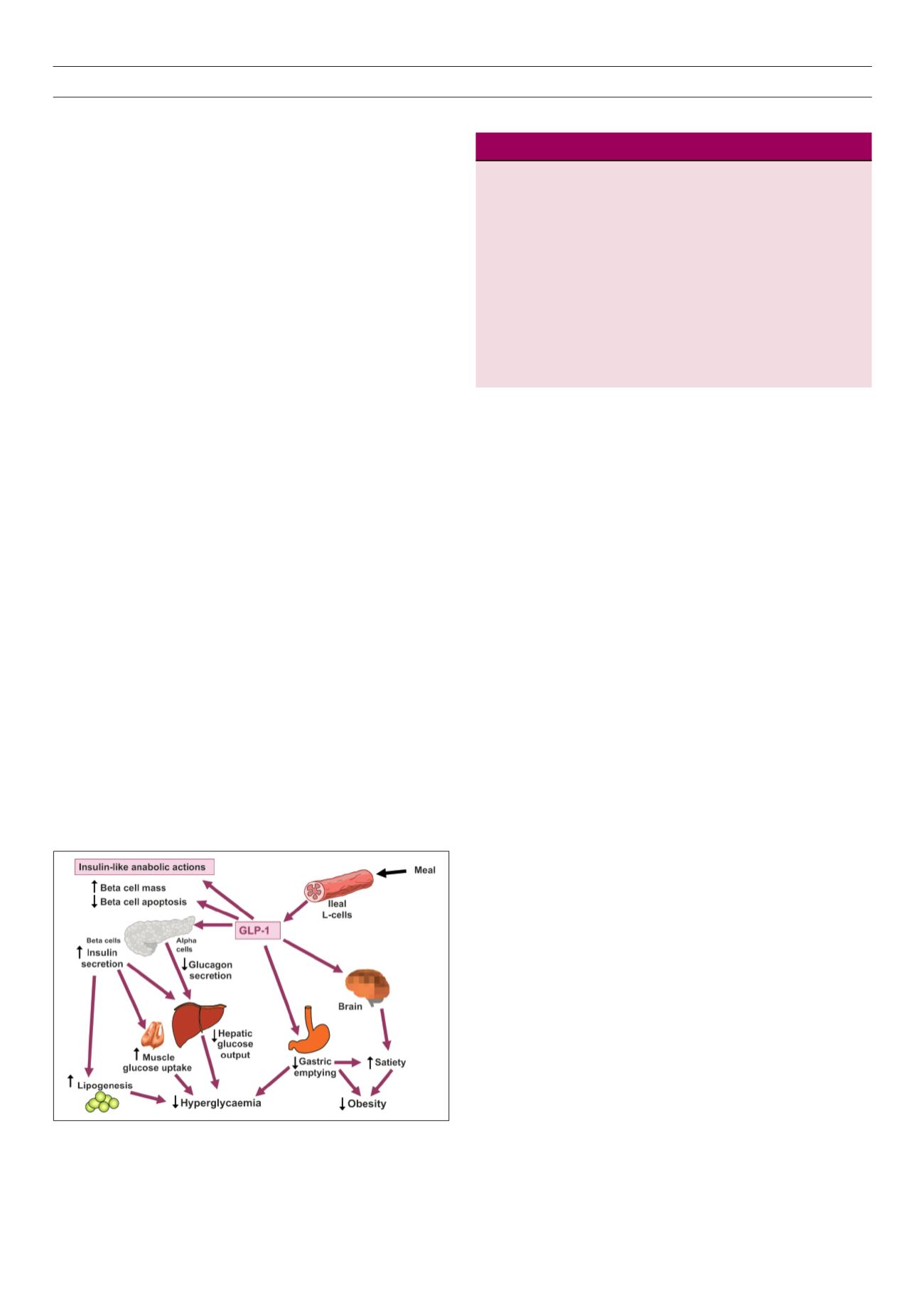
Figure 2.
Sites of action of GLP-1 to reduce hyperglycaemia and weight gain.
Source: reproduced with permission from
Br J Diabetes Vasc Dis
.15
Domain
Action of GLP-1 and GLP-1 based therapies
Vasculature
Modest improvement in systolic blood pressure.
No change in diastolic blood pressure.
18–20
Heart
Protective against ischaemic damage.
29
Possible improved prognosis in cardiac failure.
32
Brain
Enhanced satiety and reduced caloric intake.
34
Neuroprotection in animal models.
37,38
Kidneys
Enhanced sodium excretion.
43
Protection against diabetic nephropathy in animal
models.
45,46
Gastro-intestinal tract Reduced gastric motility.
47,48
Possible risk of
pancreatitis.
54–59
Thyroid
Medullary cancer in rodents. No effect in humans
67,68
Body composition
Reduced body fat mass
69,76
Table 1.
The effects of GLP-1 on major organ systems.


