REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
22
VOLUME 8 NUMBER 1 • MARCH 2011
Insulin devices: an added complexity
For biosimilar insulins, the additional dimension of the
administration device should also be considered. Stringent
regulatory requirements for insulin administration devices specify
use of durable labels and distinguishing marks, visibility of the
dose and accuracy with which it is dispensed after storage and
handling under a variety of environmental conditions, including
having been physically dropped. For example, cartridges, syringe/
needle systems, disposable and reusable pens and pumps must be
tested with each insulin formulation and concentrations that will
be used. Since the combinations of insulin and device may differ
widely in their dosing characteristics, it cannot be assumed that an
insulin biosimilar will be compatible with an existing administration
device. For this reason the EMA requires that compatibility is
demonstrated.
23,24
Insulin pen injectors and cartridges (3.0 ml
cartridge in the U-100 strength is the current market standard)
provide more accurate and reproducible dosing than syringes and
vials. They are also more convenient, easier to transport and may
improve safety and adherence,
25–27
suggesting that the availability
of pen injectors should be a requirement for insulin biosimilars.
Regulatory requirements for insulin biosimilars
In recent years the EMA has produced an overarching guideline
on biosimilars
28,29
as well as guidance documents addressing quality
issues,
30
non-clinical and clinical issues,
31
and guidelines for specific
biosimilars, including soluble recombinant human insulin.
21
As mentioned earlier
9
the EMA requires that biopharmaceuticals
undergo comprehensive comparability studies of both the drug
substance and product to provide evidence that the biosimilar is
indeed similar in quality, safety and efficacy to a single appropriately
chosen reference product that has the same pharmaceutical form,
strength and route of administration that is already approved
in the EU. In general, required preclinical data include primary
pharmacology and repeat-dose toxicology data. The EMA requires
that maintain isotonicity (to minimise injection pain and tissue
damage).
22
Any variations in the entire process of insulin synthesis and
formulation may result in a product which may be physicochemically
very similar to an appropriate reference product, but which differs
subtly in its clinical PK or PD characteristics.
9,14
The steps in the
manufacture of insulin are summarised in Fig. 6.
Figure 5.
Insulin medicinal products currently available: a summary.
Insulin medicinal products
Rapid-acting insulin formulations
Insulin analogues: insulin aspart, insulin
glulisine, insulin lispro monomers, clear
solution
Regular-acting insulin formulations
Zn- and preservatives containing insulin
hexamers clear solution
Intermediate-acting insulin formulations
Insulin or insulin analogue isophane
suspension (Cocrystallised insulin and
protramine) turbid or cloudy suspension
Long-acting insulin formulations
Ultralente insulin-Zn-crystals: turbid
suspension insulin analogues; insulin glragine,
insulin determir clear solution
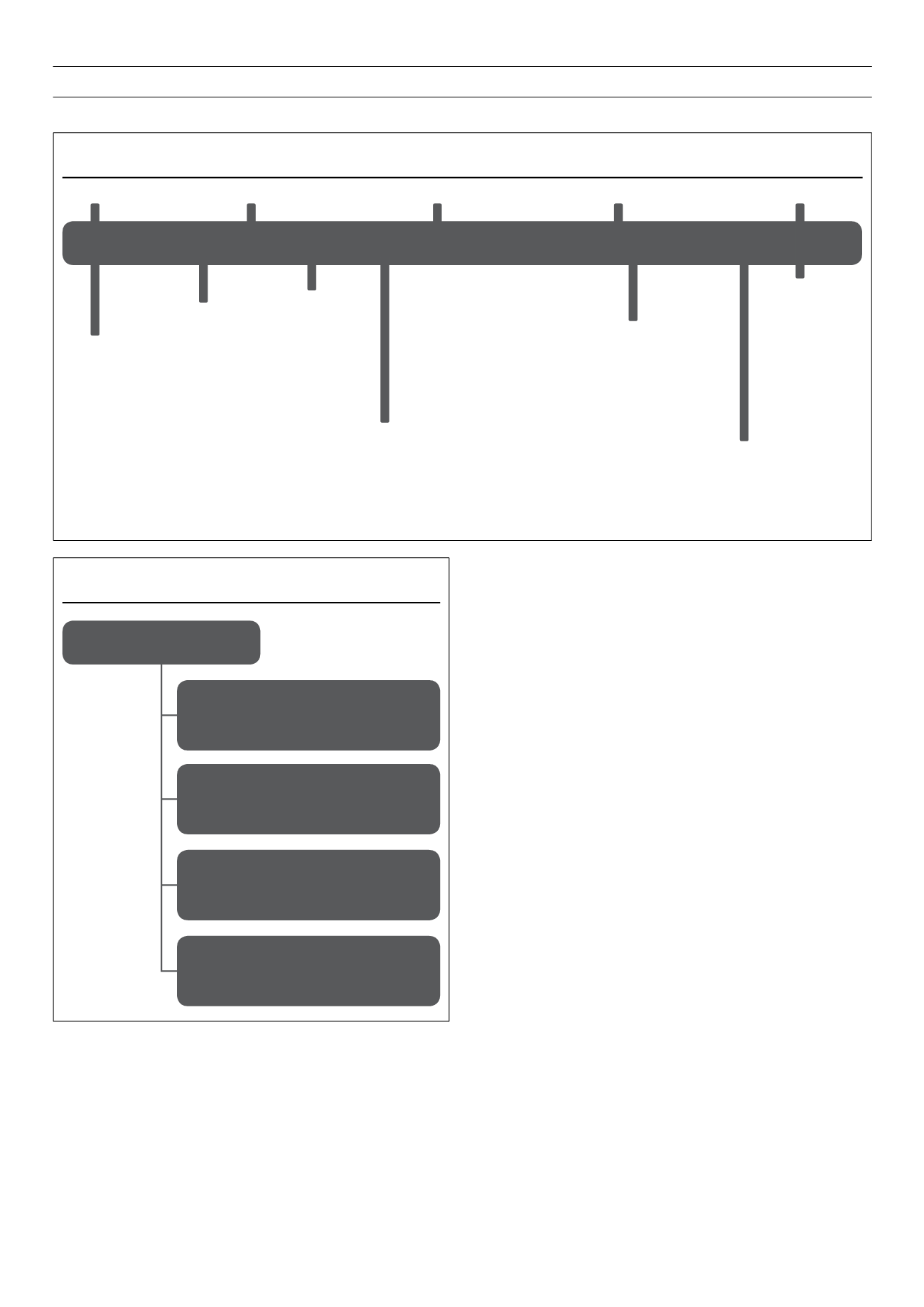
Figure 4.
The development of therapeutic insulin: a timeline.
14–16
Since the discovery of native insulin in 1921, successive discoveries have improved the production of therapeutic insulins as well as their pharmacokinetic and
pharmacodynamic properties. Work on therapeutic insulins has garnered three Nobel Prizes.
NPH
=
neutral protamine Hagedorn; PZI
=
protamine zinc insulin.
Insulin
discovered
PZI
NPH
insulin
Lente
insulins
Human
insulin
Rapid-acting
analogue
Long-acting
analogue
1921
1940
1960
1980
2000
Nobel Prize
1923
Nobel Prize
1958
Nobel Prize
1977


