SA JOURNAL OF DIABETES & VASCULAR DISEASE
REVIEW
VOLUME 8 NUMBER 1 • MARCH 2011
15
raised values for this age group would fall within the 97.5 centile of
their age-specific range. Sub-clinical hyperthyroidism is also more
common in older age groups, but its female preponderance is less
marked. The incidence of progression to overt thyrotoxicosis is
approximately 5% per year; and patients with autonomous thyroid
adenoma or nodular goitre are especially at risk.
7
The main causes of hypothyroidism and hyperthyroidism are
Hashimoto’s thyroiditis and Graves’ disease respectively, both of an
autoimmune nature. Since type 1 diabetes also has autoimmunity
as a pathophysiological detonator it is not unusual to find patients
with concomitant diabetes and thyroid dysfunction. Some genetic
factors might contribute to the co-occurrence of AITD and type
1 diabetes.
8
Moreover the association between type 1 diabetes
and AITD is considered one of the variants of the autoimmune
polyglandular syndrome. The MHC locus on chromosome 6p21 is
one of the susceptibility loci for both diseases. An odds ratio of
approximately 2 has been reported for the association of the DR3
haplotype with Graves’ disease, which is even higher, between 3
and 4, in people who have type 1 diabetes. Several other factors
that intervene in the immune response might also contribute to
AITD and type 1 diabetes susceptibility. PTPN22, which encodes
lymphoid tyrosine phosphatase, a negative regulator of T-cell
antigen receptor (CD3) signalling and the cytotoxic T-lymphocyte
antigen-4 (CTLA4) gene have both been confirmed as major joint
susceptibility genes for type 1 diabetes and AITD.
Prevalence studies show that AITD is higher in type 1 diabetes.
Perros
et al
.
9
reported thyroid dysfunction in up to 31.4% of
adult type 1 diabetic females. Moreover, in children with type 1
diabetes, the proportion of positive thyroid antibodies might
increase up to 20% and about 3–8% of children and adolescents
with type 1 diabetes have been reported to develop autoimmune
hypothyroidism.
10
Postpartum thyroiditis, a rather common event,
with an incidence of 4–6% as evident from several population-
based studies, is threefold higher (up to 25%) in women with type
1 diabetes.
11
Although thyroid disease, overt or sub-clinical, is reported to be
relatively common in type 1 diabetes, a longitudinal Australian study
in type 2 diabetic women without known thyroid disease showed
that sub-clinical hypothyroidism is a common, but incidental
finding.
12
Nevertheless, increased risk for thyroid autoimmunity in
adult type 2 diabetic patients with GAD65 autoantibodies has been
reported, and these findings have been confirmed in paediatric
populations.
13,14
As regards the metabolic syndrome, as might be
expected, the prevalence of sub-clinical hypothyroidism is higher
in patients with the condition than in non-metabolic syndrome
subjects.
15
These findings can be explained by the concomitance
of deranged serum lipid concentrations, obesity, hypertension and
insulin resistance, all components present in metabolic syndrome as
well as in hypothyroid patients.
In viewof the relatively high prevalence of both endocrinopathies,
it is important to investigate all diabetic patients for thyroid
disorders. However, screening has been recommended only in
children and adolescents with type 1 diabetes.
7,16
TSH should be
tested several weeks after the diagnosis of type 1 diabetes, when
metabolic control has been established. If the TSH level is normal,
patients should have a repeat measurement every one to two years.
Additional thyroid function testing should be obtained whenever
thyroid dysfunction is suspected or thyromegaly is detected. With
regard to diabetic adults, there is no consensus as to whether
screening for thyroid disorders should be mandatory.
Pathological mechanisms common to thyroid
disorders and diabetes
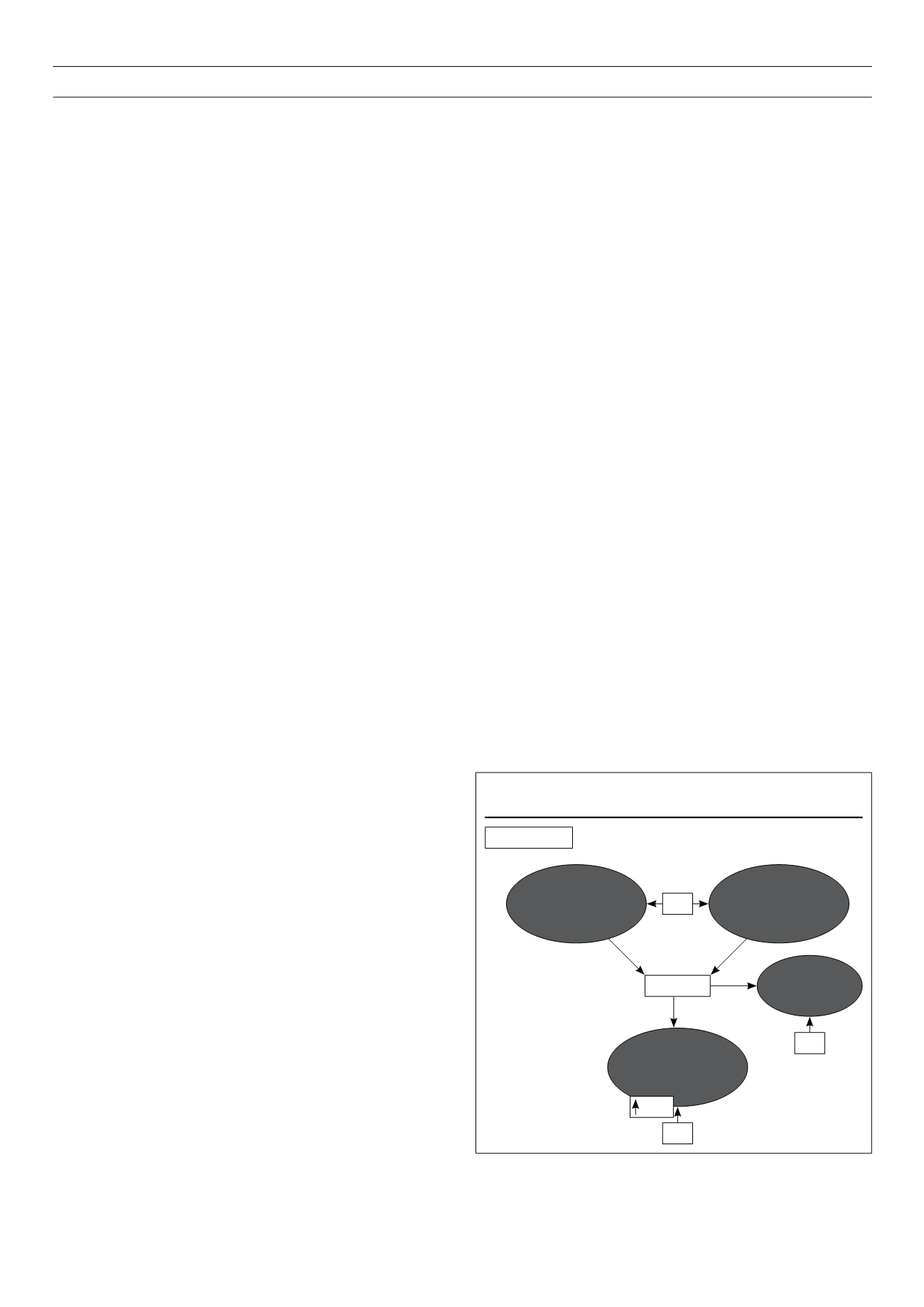
Thyroid hormones exert profound effects in the regulation of
glucose homeostasis. These effects include modifications of
circulating insulin levels and counter-regulatory hormones,
intestinal absorption, hepatic production and peripheral tissues (fat
and muscle) uptake of glucose (Fig. 1). It has long been known
that thyroid hormones act differentially in liver, skeletal muscle and
adipose tissue – the main targets of insulin action. While thyroid
hormones oppose the action of insulin and stimulate hepatic
gluconeogenesis and glycogenolysis,
18,19
they up-regulate the
expression of genes such as GLUT-4 and phosphoglycerate kinase,
involved in glucose transport and glycolysis respectively, thus acting
synergistically with insulin
20,21
in facilitating glucose disposal and
utilisation in peripheral tissues. The recent identification of another
gene regulated by thyroid hormones in cultured human fibroblasts,
22
the transcription factor HIF-1
a
, responsible for elevated expression
of glycolytic enzymes and glucose transporters, is an example that
the field of thyroid diabetes is still open to new discoveries.
Thyroid disorders have a major impact on glucose control. When
thyroid dysfunction ensues the glucose homeostatic balance is
broken (Fig. 2). Insulin resistance, mainly associated with increased
hepatic gluconeogenesis, is characteristic of an excess of thyroid
hormones and explains why glucose control deteriorates when
diabetic patients develop hyperthyroidism. Thyrotoxic patients show
an increased glucose turnover with increased glucose absorption
through the gastrointestinal tract, post-absorptive hyperglycaemia
and elevated hepatic glucose output, along with elevated fasting
or postprandial insulin and proinsulin levels, elevated free fatty
acid concentrations and elevated peripheral glucose transport and
utilisation. In peripheral tissues there is a massive arrival of glucose
to the cells that overwhelms the Krebs cycle resulting in an increased
metabolism of glucose through the nonoxidative pathway. Lactate
produced in great quantities in the cells returns to the liver and
participates in the Cori cycle where four ATP molecules are wasted
for each glucose molecule that is created.
23
Figure 1.
Thyroid hormone (TH) effects on glucose homeostasis.
Increased beta-
cell function
Increased peripheral
tissues glucose utilisation
(insulin synergism)
Euthyroidism
TH
Glucose
TH
TH
Increased
intestinal
glucose
absorption
Increased hepatic
gluconeogenesis
glycogenolysis
(insulin antagonism)
Glut 4


