SA JOURNAL OF DIABETES & VASCULAR DISEASE
REVIEW
VOLUME 8 NUMBER 1 • MARCH 2011
21
or substituted amino acid residues. The first short-acting analogues,
insulin lispro and insulin aspart, were introduced in the late 1990s,
and the first long-acting analogue insulin glargine in 2000. Each
of these innovations was made with a view to improved onset and
duration of action,
14–16
to reduce hypoglycaemia and hyperglycaemia
and to improve tolerability. The history of developments in insulin
is summarised in Fig. 4 and a summary of available insulins is given
in Fig. 5.
In the manufacture of recombinant human insulin, the
recombinant organism that actually expresses the precursor protein
is generally
Escherichia coli
or a yeast such as
Saccharomyces
cerevisiae
. The engineered gene encoding for the precursor protein
must be inserted into a suitable stable expression vector. The choice
and the characteristics of this construct will affect key aspects, such
as the degradation characteristics of soluble proteins and the yield
of the process. The recombinant cells are screened, and a well
characterised master cell bank is established from a single clone.
This master cell bank is used to create uniform working cell banks
that are used to cultivate the cells and produce the desired product.
During product synthesis the culture and fermentation conditions
are tightly controlled in order to optimise yields and avoid formation
of unwanted by-products.
17
Generally, impurities come from either
the growth medium (especially for products isolated from cell
culture supernatant) or the host cells. These impurities can be host-
related (e.g. endotoxins, HCPs, DNA, viruses), product-related (e.g.
denatured protein, aggregates, protein fragments, deamidated
species, conformational isomers), or process-related (e.g. growth
medium components, metals, column material). When the product
is recovered, modified and purified the formation of inclusion
bodies (for example in high-yield
E. coli
processes), requires the
disruption of the cells to release preproinsulin. This is then isolated,
purified and folded, and then enzymatically cleaved to produce the
mature insulin molecule.
18–20
In the case of insulin, impurities such
as desamido forms may arise as by-products of conversion from
proinsulin to insulin by removal of the C-peptide and regeneration
of the three-dimensional form of the molecule.
21
After numerous purification steps, the insulin is crystallised
or lyophilised and formulated. The insulin molecule is negatively
charged at neutral pH, and readily associates into dimeric complexes
or into zinc-containing hexamers (Fig. 2). Thus, zinc may be added
to trigger aggregation into soluble discrete hexameric structures
containing two zinc ions per hexamer.
14
Phenolic excipients, added
as antimicrobial agents, also bind to specific sites on hexameric
insulin, changing its conformation to a more stable form (so-called
T–R transition). Other agents added at the formulation stage
may include physiological buffers (to maintain pH) and agents
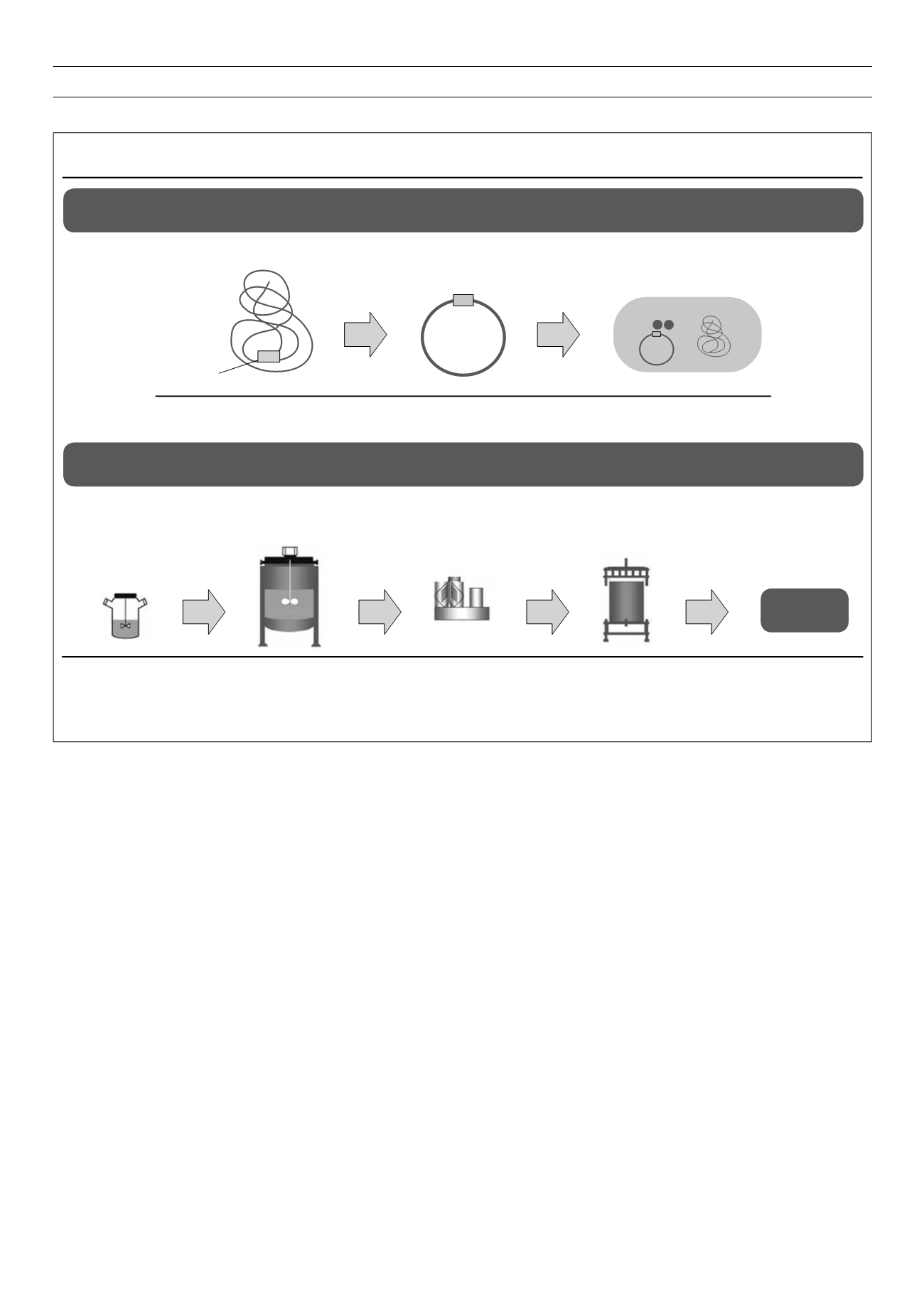
Figure 3.
Manufacture of a biopharmaceutical: opportunities for variation between manufacturers.
11
Adapted from Mellstedt H,
et al
.
Ann Oncol
2008;
19
: 411–9.
11
Cloning and protein expression
Cloning into DNA vector
Transfer into host cell
expression screening/selection
Source
DNA
Target DNA
Possibly same
gene sequence
Probably
different vector
Different cell
expression system
Protein production, purification and validation
Cell
expansion
Cell production
in bioreactors
Recovery through
filtration or
centrifugation
Purification through
chromatography
Characterisation
and stability
Different cell line,
growth media,
method of expansion
Different cell line,
growth media,
bioreaction conditions
Different
operating
conditions
Different binding
and elution
conditions
Different methods,
reagents, reference
standards
Purified
bulk drug


