REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
12
VOLUME 8 NUMBER 1 • MARCH 2011
a well-defined genetic susceptibility. This is supported by numerous
factors, including that the concordance rate of T1D in monozygotic
twins is only about 40% and the risk for a sibling to develop T1D is
4% at 20 years age and 9.6% by 60 years age. T1D is two to three
times more common in offspring of a diabetic man compared to a
diabetic mother.
4
A child with both of the highest-risk haplotypes (DR3-DQ2
and DR4-DQ8) has a 5% chance of developing T1D by the age
of 15 years but if that child has a sibling with the same haplotype
and diabetes, the risk increases to about 55% compared to a
population incidence of 0.5%.
7,8
The high-risk haplotype is present
in about 40% of children with T1D compared to about 2% in the
general population.
8
Table 2 lists the high-risk and protective HLA
haplotypes.
8
Fig. 1 represents the odds ratios and probable role of
the non-HLA loci in T1D susceptibily.
7
Andrew Hattersley states that a molecular diagnosis of diabetes
is possible for most patients.
6
This may be true in developed
countries but not so in South Africa with our limited access to
genetic testing. Immunological and genetic determination of the
HLA haplotyping is available in South Africa, as well as genetic
testing for some South African genetic diseases (CF, PWS, BBS)
associated with diabetes. No laboratory offers routine analysis for
the MODY, insulin-dependent or other genetic types of diabetes.
We can therefore only use HLA haplotyping and the presence of
auto-antibodies to refine the risk of developing T1D.
It is generally accepted that an environmental trigger is required
to initiate the autoimmune process that causes type 1A diabetes.
Apart from congenital rubella, no other environmental agent has
yet been convincingly shown to result in T1D.
4,5,7,8,15
However, the
involvement of
IFIH1
in the innate immune recognition of picorna
virus
7
may add weight to theory of a viral trigger.
The above figures form the basis of calculating the risk for
developing T1D. Additional information can be obtained by
testing for the presence of antibodies to pancreatic islet cells,
insulin, glutamic acid decarboxylase (GAD) and protein tyrosinase
phosphate (IA-2).
16
The presence of antibodies does not always
indicate the presence of
β
-cell destruction. Infants of type 1A
diabetic mothers, who have GAD65 and IA-2 antibodies at birth
but no insulin antibodies, have a reduced development of anti-islet
antibodies and do not develop diabetes.
4,5
The HLA haplotype DQA1*0102DQB1*0602 is protective, with
a markedly reduced association with diabetes.
5,8
HLA haplotyping
of black South Africans has to the author’s knowledge not been
undertaken and therefore the risks associated with HLA haplotypes
may need to be used with some caution, even though the HLA
association has been confirmed in many different populations and
ethnic groups across the globe.
Using available genetic and aetiological information, prevention
strategies have been undertaken in animal models. To date, safe
prevention in humans has not been achieved but trials aimed
at preventing diabetes are being undertaken. These trials target
the prevention of the autoimmune process being initiated, or
strategies to preserve pancreatic
β
-cells after diagnosis. The lack
of precise and cost-effective identification of high-risk individuals
means that large numbers of subjects need to undergo potentially
harmful treatment to prevent a small amount of diabetes. Starting
preventative strategies later will target persons with a more
predictable risk but reduce the number of potential strategies and
impact.
16
Counselling on the course of the disease and lifestyle adaptation
for T1D should be undertaken by diabetologists and dieticians
experienced in the care of people with diabetes, rather than by
medical geneticists or genetic counsellors. The same is probably
true for regular screening of a person at increased risk of
developing diabetes. Should lifestyle changes or other prevention
strategies become available to offset the development of diabetes,
this will probably also be part of the duties of a diabetologist or
dietician experienced in the care of people with diabetes. Diabetic
care, diagnosis, treatment and counselling is generally outside the
expertise of medical geneticists and genetic counsellors, who will
only encounter the condition when confronted with people with a
syndrome in which diabetes is part of the phenotype.
Complications of diabetes
Genetic markers that predict the development of specific
complications of diabetes, such as nephropathy,
17
retinopathy or
vascular disease, are also available.
18
The value of these markers is
probably influenced by ethnicity but holds great promise for the
practise of ‘personalised medicine’ in those persons with T1D,
to modify the care that a specific patient receives to alleviate the
problems of these complications.
Conclusion
There have been major advances in the understanding of the
aetiology, pathogenesis and genetics of T1D in recent years.
Risk calculation and genetic counselling for people with or at
risk of developing T1D is certainly no longer a nightmare but is
still challenging and needs close attention to detail of the many
different aspects of this complex disease. At least for the present,
this should remain the responsibility of those with the relevant
knowledge and experience to undertake the task, diabetologists
and dieticians experienced in the care of people with diabetes.
Acknowledgements
The author thanks Profs A Christianson and W Mollentze for their
encouragement and positive comments during the development of
this manuscript.
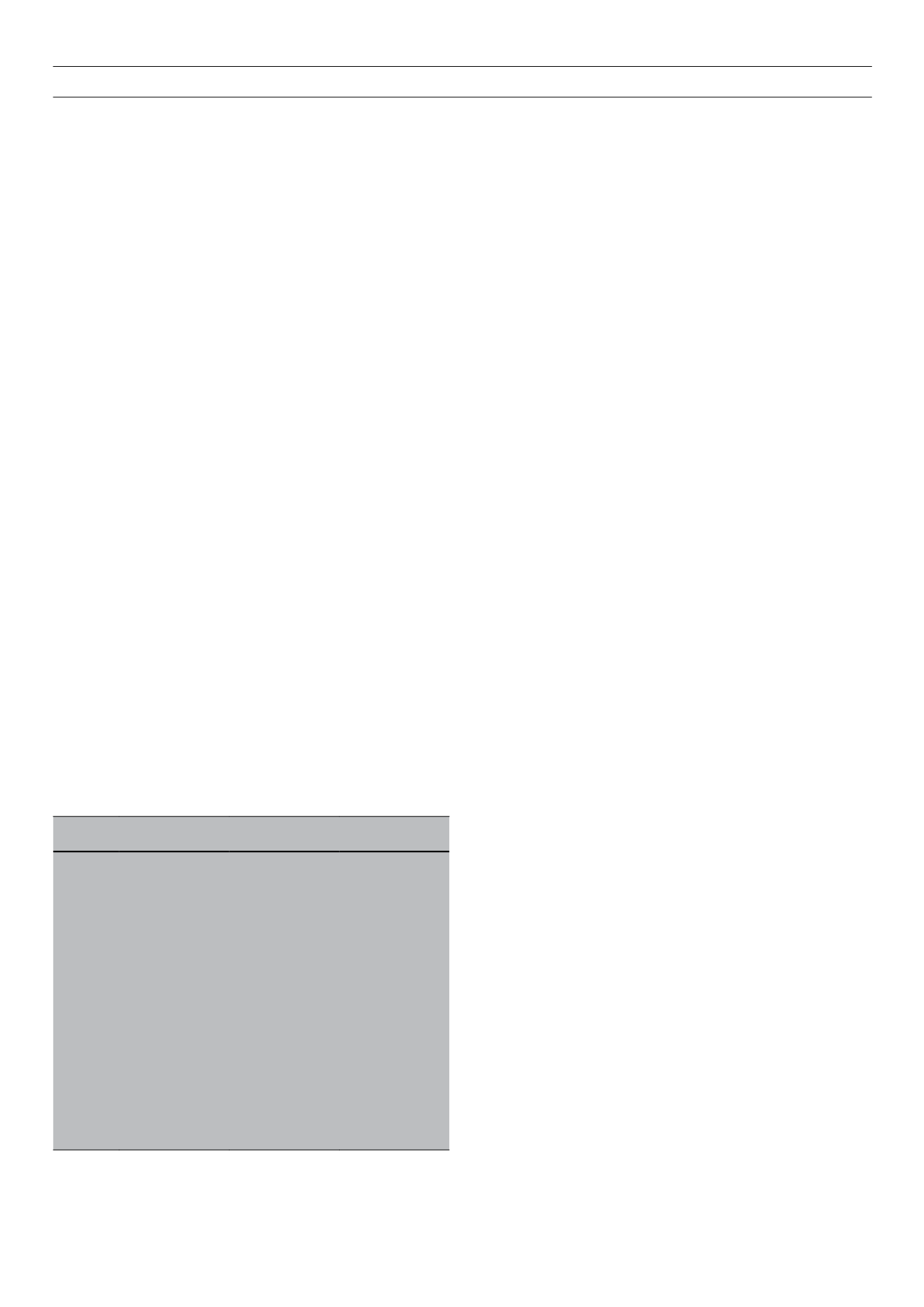
Table 2.
The HLA haplotypes associated with T1D
High-risk haplotypes
DR3
DRB1*0301
DQA1*0501
DQB1*0201
DR4
DRB1*0401
DQA1*0301
DQB1*0302
DRB1*0402
DQA1*0301
DQB1*0302
DRB1*0405
DQA1*0301
DQB1*0302
Moderate-risk haplotypes
DR1
DRB1*01
DQA1*0101
DQB1*0501
DR8
DRB1*0801
DQA1*0401
DQB1*0402
DR9
DRB1*0901
DQA1*0301
DQB1*0303
Strongly protective haplotypes
DR2
DRB1*1501
DQA1*0102
DQB1*0602
DR6
DRB1*1401
DQA1*0101
DQB1*0503
DR7
DRB1*0701
DQA1*0201
DQB1*0303


