REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
16
VOLUME 8 NUMBER 1 • MARCH 2011
Although glucose uptake in peripheral tissues has been described
as either normal or increased,
24,25
reduced insulin stimulated
peripheral glucose utilisation has also been demonstrated in
hyperthyroidism.
26
The notion that insulin stimulation of glucose
uptake in thyrotoxic tissues may be impaired can be interpreted in
the context of lower glucose extraction from serum in proportion to
increased blood flow.
27
As regards insulin secretion, thyrotoxicosis
has been associated with normal, decreased or increased beta-cell
function.
2,28
However, it has been suggested that proinsulin in excess
may account for the hyperinsulinemia observed with higher release
of insulin both after absorption and at baseline, when compared
with the euthyroid situation or with control subjects.
2
Moreover,
recent studies have shown that thyroid hormones increase beta-cell
apoptosis and that this could be one major element responsible for
deterioration of glucose tolerance in thyrotoxicosis.
2,29
In hypothyroidism, glucose homeostasis is also affected
although its clinical impact is less obvious (Fig. 3). Decreased
glucose disposal (compared with euthyroid subjects) has been
proved in hypothyroid patients by different methods including
clamp studies,
30,31
the arteriovenous difference technique in the
anterior abdominal subcutaneous adipose tissue and forearm
muscles after the consumption of a mixed meal,
32
the insulin
tolerance test
33
and following intravenous
34
or oral
35
administration
of glucose. Nonetheless, hypothyroidism results in unimpaired
36
or decreased
37,38
liver glucose output thereby compensating for
insulin resistance present in peripheral tissues and accounting
for the diminished insulin requirement for glycaemic control in
hypothyroid diabetic patients. With regard to beta-cell function,
normal or reduced basal plasma insulin levels have been described
in hypothyroidism. These findings are quite consistent with the idea
of attenuated endogenous glucose production in the hypothyroid
state.
2
On the other hand, increased glucose-stimulated insulin
secretion has been recently described in humans and interpreted
as a response to elevated whole-body insulin resistance increasing
demand on beta-cells.
31
Although most of these observations apply to overt
hypothyroidism, insulin resistance has been also reported in sub-
clinical hypothyroidism,
35
adding one more possible mechanism to
the association of sub-clinical hypothyroidism and cardiovascular
risk. Furthermore, it has been shown, both in euthyroid non-
diabetic
39
and diabetic adults,
40
that small variations in TSH at
different levels of insulin sensitivity might exert a marked effect on
lipid levels. The interaction between insulin resistance and lower
thyroid function might be a key determinant for a more atherogenic
lipid profile in these populations (Fig. 4).
Even though thyroid status, as assessed by plasma hormone
levels, is a key indicator of glucose homeostasis, T3 intracellular
pathways are also relevant. The hormonal message is modulated at
a local level by a series of control steps, including the intracellular
concentration of T3 via deiodinases, and the relative concentration
of T3 receptor isoforms, co-activators, and co-repressors. These
systems ultimately result in tissue-specific thyroid hormone action,
which is relatively independent of the circulating thyroid hormone
levels. Polymorphism Thr92Ala, which confers a lower activity to
type 2 deiodinase, has been associated with insulin resistance in
some populations
41
and is a good example of hidden regulatory
mechanisms.
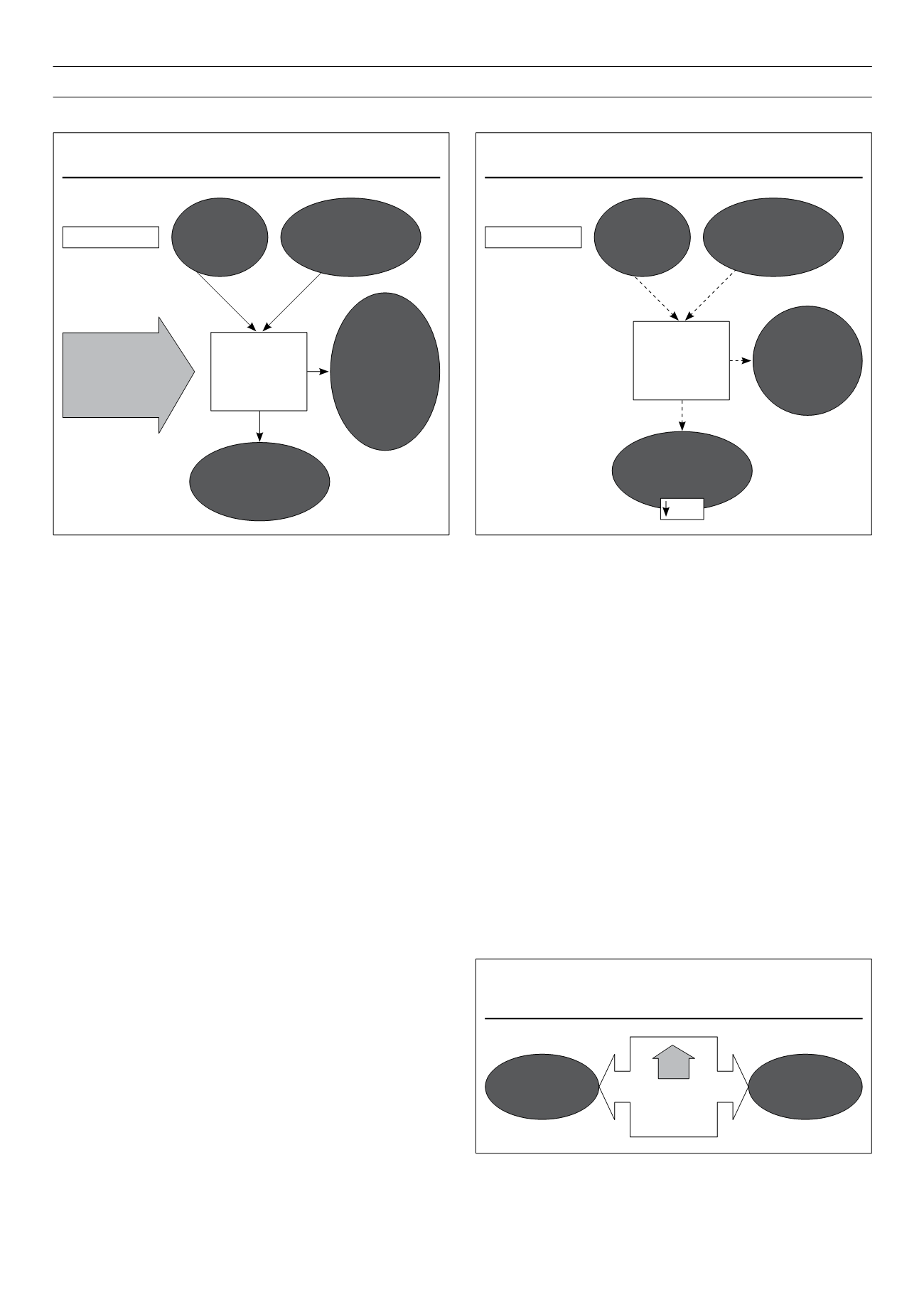
Figure 2.
Thyrotoxicosis and glucose homeostasis.
Increased
peripheral tissues
glucose utilisation
with peripheral
insulin resistance
Hyperthyroidism
Increased
hepatic glucose
output and
postabsorptive
glycaemia
Highly
increased
intestinal
glucose
absorption
Increased hepatic
gluconeogenesis
glycogenolysis with
hepatic insulin
resistance
Elevated
fasting and/or
postprandial
insulin (at
the expense
of proinsulin/
inactive) levels.
Apoptosis
of insulin-
producing
cells
Thyrotoxicosis
results in glucose
intolerance and
increased insulin
requirements in
diabetes
Figure 3.
Hypothyroidism and glucose homeostasis.
Decreased
peripheral tissues
glucose disposal
Hyperthyroidism
Reduced
hepatic glucose
output and
postabsorptive
glycaemia
Decreased
intestinal
glucose
absorption
Decreased hepatic
gluconeogenesis
glycogenolysis
Reduced
baseline
plasma insulin
levels with
increased post
glucose insulin
secretion
Glut 4
Figure 4.
Relationship between serum thyroid stimulating hormone (TSH)
and cholesterol appears to be modified by insulin resistance (IR).
In euthyroid
diabetic and
non-diabetic
subjects
Lipid parameters
with adverse
cardiac risks
Lower risk for
dyslipidaemia
TSH


