SA JOURNAL OF DIABETES & VASCULAR DISEASE
REVIEW
VOLUME 8 NUMBER 1 • MARCH 2011
23
toxicological studies that focus on potential immunogenicity,
as well as
in-vitro
affinity bioassays, assays for insulin and IGF-1
receptor binding, and tests for intrinsic activity.
28–31
One of the main concerns when switching or substituting
insulin products is hypoglycaemia caused by differences in activity
of different brands. Therefore, it is obligatory to ensure that the
effects of any insulin product in clinical use are highly consistent
and predictable. The EMA requires at least one PK single-dose
crossover study that compares the biosimilar insulin with the
reference product, using subcutaneous administration, preferably
in patients with type 1 diabetes. Clinical activity must be determined
in a comparative PD study, designed as a double-blind, crossover,
hyperinsulinaemic, euglycaemic clamp study, to demonstrate the
product’s hypoglycaemic response profile. Current EMA guidelines
for soluble insulin biosimilars do not require a clinical efficacy trial,
but do require a clinical safety study. The product’s immunogenicity
must be investigated through clinical studies of at least 12 months,
including a comparative phase lasting at least 6 months. Finally, the
manufacturer must also design a pharmacovigilance programme
that will rapidly detect any clinically significant immunogenicity
that may emerge over extended time periods.
21,28–30
The application
for marketing authorisation of three biosimilar insulin formulations
in March 2007 suggested deficiencies in long-term efficacy and
inadequate immunogenicity testing.
32–34
The details of these applications were recently reviewed by
Kuhlmann and Marre in this journal.
35
What clinicians should know before selecting a
biosimilar insulin
When contemplating biosimilar insulins, it is important to
consider the manufacturer, protein quality and formulation, batch
consistency and reliability of supply (Table 1).
36,37
Reassurance can
be gained from full disclosure of information to the healthcare
community about the manufacturing process and about safety
testing.
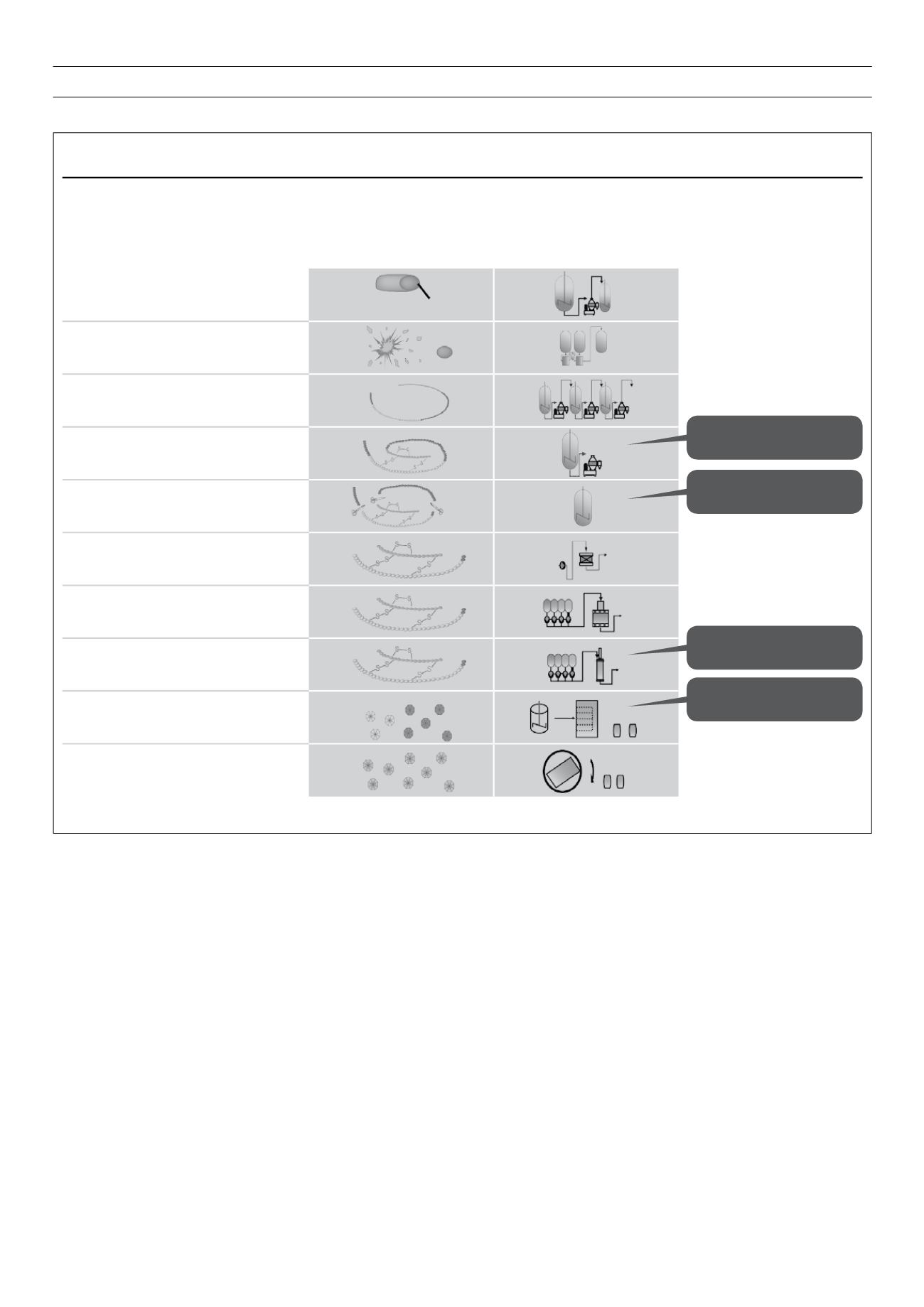
Figure 6.
A highly complex process: steps in the manufacture of insulin.
Reproduced with kind permission from sanofi-aventis group.
Insulin glargine:
Manufacturing is a complex, multi-step process
By-product profile influenced by folding and cleavage
Isolation of cells
Cell disruption via homogeniser
Isolation and Purification
Folding
Enzymatic cleavage
Prepurification and concentration via
absorption
Ion-exchange chromatography
Reversed-phase chromatography
Crystallisation and lyophilisation
Blending/filling
Typical operations
for inclusion
body processes
Folding conditions influence
by-product pattern
Cleavage enzyme:
specificity and selectivity
Chromatography defines
purity
Fibrillation tendency
E.coli
cell
Inclusion body
Fusion protein
Preproinsulin
Arg-Insulin
Insulin Glargine


