REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
20
VOLUME 8 NUMBER 1 • MARCH 2011
proteolytically cleaved to produce active insulin (Fig. 1). The insulin
preparation must also be formulated to control the formation
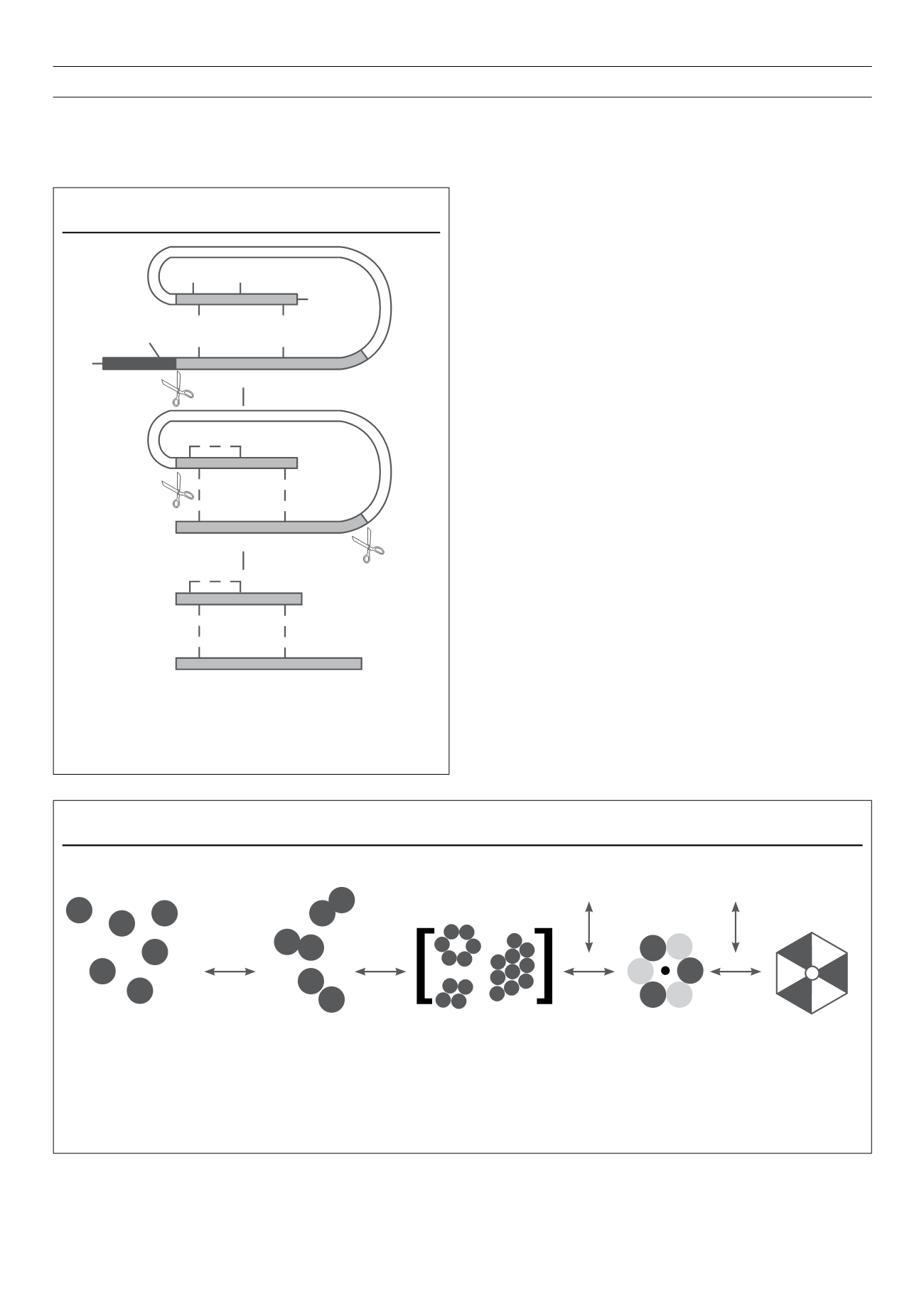
of discrete hexamers (complexes of six insulin monomers), to
confer the required absorption characteristics (Fig. 2). The more
recent insulin analogues contain additional or substituted amino
acid residues or other functional groups introduced by genetic
engineering or by biochemical modification. These changes alter
the speed of bioavailability and thereby modify the PK and PD
profiles of the molecule.
Given their structural complexities biopharmaceuticals are
far more difficult to manufacture than small-molecule drugs.
The use of living organisms introduces an inherent variability
in the manufacturing process. To guarantee the quality and
compliance of each production batch, absolute consistency of the
manufacturing process is required. Even apparently slight changes
in any of the manufacturing or formulation steps can have major
clinical consequences.
4–8
Manufacturers of biosimilar products
must develop proprietary expression systems and processing steps
independently. Thus biopharmaceuticals can never be identical
copies of originator molecules, even when they have demonstrated
comparable physicochemical and biological properties to a
reference product using currently available tests. The most they can
achieve is ‘biosimilarity’.
5,9,10
For non-glycosylated products such as
human insulin, PK and PD differences are most probably caused
by differences in formulation, while for glycosylated products (e.g.
epoetin), the glycosylation pattern is probably the major source
of PK/PD variations. The essential steps in the manufacture of a
biopharmaceutical and the loci of potential variability in their
manufacturing processes are shown in Fig. 3.
11
The first commercial insulins, extracted from beef and pork
pancreata, became available shortly after the discovery of insulin
in 1921, and the first long-acting insulins, PZI and NPH, had been
developed by the 1940s. However, patients using animal insulin
products that were not highly purified often had local injection site
reactions or more rarely, systemic reactions such as IgE-mediated
anaphylaxis.
12
Highly purified products and recombinant insulins
are associated with decreased levels of anti-insulin antibodies.
Nonetheless, there is little evidence that antibody formation affects
glucose control or causes other complications of insulin therapy.
13
The more recent recombinant insulin analogues contain additional
Figure 1.
The biosynthesis of insulin.
The insulin precursor preproinsulin contains a signal sequence that is
proteolytically cleaved to yield proinsulin, whose C chain links the future A
and B chains of mature insulin. Cleavage of the C chain converts proinsulin
to insulin.
Adapted from Joshi SR,
et al
.
J Assoc Physicians India
2007;
55
(suppl): 19–25.
14
C-Peptide
SH SH
SH
SH
SH
SH
COO –
Signal sequence
Preproinsulin
H
3
N
Signal peptidase
cleavage
Tryptic
cleavage
A Chain
B Chain
C-Peptide
S S
S
A Chain
B Chain
Proinsulin
S
S S
Tryptic
cleavage
S S
S
A Chain
B Chain
Insulin
S
S S
Figure 2.
Association of insulin monomers in the presence and absence of zinc and phenolic excipients.
Insulin readily associates into dimers, aggregates and (in the presence of divalent cations such as zinc), into hexameric forms. The presence of phenolic excipients
causes these hexamers to undergo conformational changes that increase their stability.
R6
=
hexamer with insulin molecules whose B1–B8 residues are in an
α
-helical (R) conformation; T6
=
hexamer with insulin molecules whose B1–B8 resi-dues are
in an extended (T) conformation.
Adapted from Beals,
et al
. Informa Healthcare: New York, 2008; 265–80.
22
Insulin
monomer
Insulin
dimer
Insulin
aggregates
Insulin
hexamer (T6)
Insulin
hexamer (R6)
Phenolic
preservative
Zn
2+


