
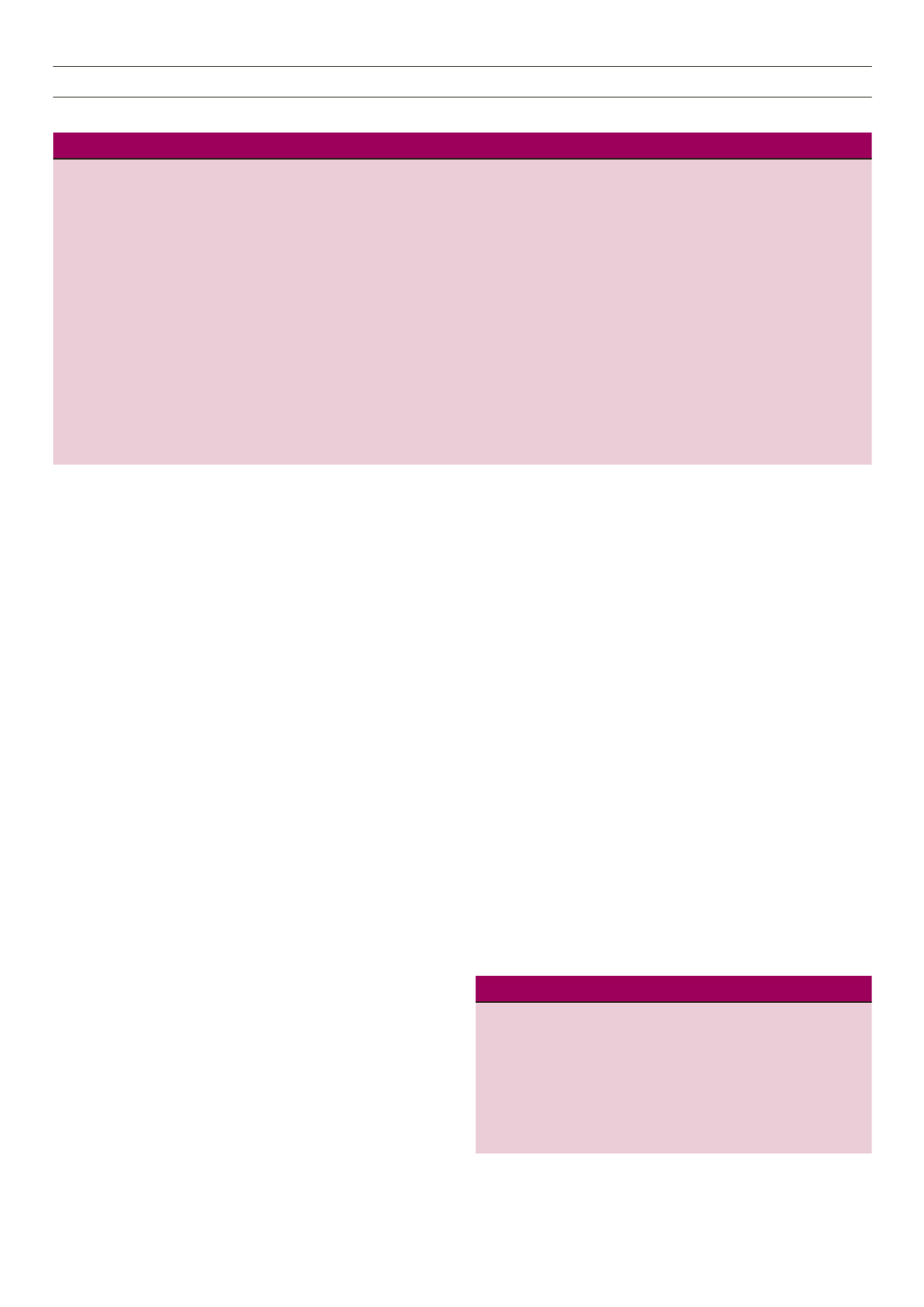
VOLUME 17 NUMBER 1 • JULY 2020
31
SA JOURNAL OF DIABETES & VASCULAR DISEASE
CASE REPORT
pharmacological treatment. Insulin-induced hypokalaemia results in
a decrease in serum potassium level due to intracellular potassium
shifts and, potentially, the aldosteronelike effect of insulin on the
renal tubule further increases urinary potassium losses.
The goal of the treatment for insulin-induced hypokalaemia
(K
+
< 2.5 mmol/l) is to replenish potassium stores through slow
intravenous infusion of KCl,
6
with insulin therapy delayed until
serum potassium levels are corrected back to > 2.5 mmol/l.
7
The
most severe complication of hypokalaemia is lethal arrhythmia, such
as VT/Vf. Potassium replenishment and cardioversion defibrillation
should be performed immediately.
In our case, the patient experienced in-hospital cardiac arrest
(IHCA) resulting from hypokalaemia-induced VT/Vf. Extracorporeal
CPR (ECPR) restored tissue and end-organ perfusion to allow
stabilisation and recovery of function. ECPR can be defined as
the implantation of VA-ECMO in a patient who has experienced
a sudden and unexpected pulseless condition attributable to
cessation of cardiac mechanical activity.
8
Many prospective
and retrospective studies have demonstrated the superiority of
ECPR over conventional CPR regarding the odds of survival and
neurological outcome.
9–11
ECPR can be viewed as a late intervention
in a moribund patient, possibly a candidate for an earlier circulatory
support system in case of IHCA.
Compared with ECMO, which provides both cardiac and
pulmonary support, a Bi-VAD usually provides cardiac support
only. However, a Bi-VAD can be implemented long term with more
cardiac support than ECMO, especially when the ECMO is set up
peripherally. Moreover, patients on ECMO support usually require
large doses of inotropes, which cause extreme vasoconstriction
and lead to malperfusion of the visceral organs. In patients with
refractory cardiogenic shock, a VAD has been reported to provide a
better survival rate than VA-ECMO.
12
In the current case, although VA-ECMO was instituted for
mechanical circulatory support and the potassium level was
corrected back to the normal range, the patient experienced
cardiogenic shock with multiple organ dysfunction and
exacerbations. Therefore, ECMO was substituted with Bi-VAD
implantation for optimal systemic perfusion. More importantly, the
Bi-VAD completely unloaded the bilateral ventricle, maximising the
likelihood of recovery from myocardial stunning.
13
Based on our
experience, the indications for VAD intervention can be defined for
these critical patients with ECMO support (Table 2).
In our case, following Bi-VAD implantation, we were able to
immediately withdraw the inotropes and all the visceral organs
were preserved. Bedside echocardiography showed no distention
of the bilateral ventricle. Initially, the pulse pressure was narrowed
but returned three days later, which implied that the myocardial
stunning was completely resolved.
The CentriMag VAD (Levitronix LLC) was chosen for several
reasons. First, it has continuous flow, which is reported to have
better outcomes than pulsatile flow, especially for lower incidence of
bleeding and thromboembolism.
14,15
Second, Levitronix CentriMag
VAD was used as a temporary short-term VAD as a bridge towards
recovery and transplantation, if not the destination. Unlike with a
long-term VAD, it is easy to implant the device without extensively
damaging themyocardium. More crucially, repairing the cannulation
sites during explanation of the VAD is simple. Third, from the
economic perspective, it is much cheaper than a permanent long-
term VAD such as the HeartMate and HeartWare devices. Fourth,
after CPR, most patients develop pulmonary oedema and poor
oxygenation, and an oxygenator is always required for optimal
oxygenation. The Levitronix CentriMag VAD, categorised as an
extracorporeal VAD, can be easily integrated with an oxygenator,
which is not possible with an intracorporeal VAD.
Table 1.
Biochemistry data, inotrope dosage and echocardiography presentation during the VAD course
Day 3
Before
after
VAD
POD1
POD2
POD3
POD4
POD5
POD7
POD11 removal
K
+
(mmol/l)
1.6
4.2
4.7
3.7
3.5
3.5
3.3
3.5
3.1
BNP (pg/ml)
176
CK (U/l)
4862
> 10000
> 10000
> 10000
3292
Tro-I (ng/ml)
8.28
7.11
5.765
3.813
1.516
BUN (mg/dl)
60
26
28
31
61
78
Cr (mg/dl)
4.2
2.5
2.6
2.1
3.7
3.0
Urine output (ml/day)
170
995
1720
1620
3060
3420
4160
1480
2295
(haemodyalysis) (haemodyalysis) (haemodyalysis) (haemodyalysis) (haemodyalysis) (haemodyalysis)
Norepinephrine (mcg/kg/min) 26.5
14.4
12.8
2.65
–
–
–
–
–
Dopamine (mcg/kg/min)
17.3
9.4
9.35
8.7
8.65
8.65
8.65
8.65
–
Epinephrine (mcg/kg/min)
16.7
13.3
13
2.7
–
–
–
–
–
L-VAD (rpm/flow)
3700/4.74
3700/5.07
3700/4.86
3600/4.5
3500/4.14
3400/3.81
2100/1.30
–
R-VAD (rpm/flow)
3000/4.87
3000/5.02
2700/4.4
2600/4.2
2400/3.75
2200/3.31
1200/0.91
–
MAP (mmHg)
65
65–75
65–75
80–90
78–86
88–100
97–105
72–82 95–100
Echocardiography LVEF (%) 10–15
30–35
51
POD = post-operative day; L-VAD = left ventricular assist device; R-VAD = right ventricular assist device, BUN = blood urea nitrogen; CK = creatinine kinase.
Table 2.
Indications of VAD intervention after ECMO support
1 ECMO flow insufficiency; ECMO complications
2 Any organ dysfunction with ECMO maximal flow
3 Three or more inotropes or large dose
4 Narrow pulse pressure, ≥ 10 mmHg
5 Sustained VT resulted from LV distension
6 Echocardiography:
No opening of aortic valve
LV thrombus formation
Blood stasis in LV, presented as smoke swirl sign



















