24
VOLUME 10 NUMBER 1 • MARCH 2013
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
had neuropathy, 47.3% had distal neuropathy, and 31.7%
had carpal tunnel syndrome. In other studies the prevalence of
autonomic neuropathy was 16 to 75%.
Progression of the neuropathy usually presents as a change in
sensation, followed by abnormal reflexes, then muscle weakness,
pain, progressive debilitating symptoms, and finally nerve death.
Diabetic peripheral neuropathy can affect any nerve and the clinical
symptoms and signs depend on which nerve or nerves have been
affected. The neuropathy can be focal, e.g. cranial nerve palsies
or entrapment neuropathy; multifocal, e.g. polyradiculopathy and
mononeuritis multiplex; or diffuse and symmetrical.
Sensory symptoms include parasthesias, hyperalgesia, allodynia,
and a burning or lancinating pain. The pain is usually burning in
nature and is worse at night. The pain typically persists for years,
causing considerable disability for the patient. Loss of sensation
or numbness and loss of proprioception often occurs and may
eventually predominate. The signs of neuropathy include diminished
vibratory perception, decreased knee and ankle reflexes, reduced
sensation to hot and cold, diminished sensation to pinprick and loss
of proprioception (Table 1).
Risk of painful neuropathy in diabetes (Table 2)
Painful neuropathy is a common and often progressive compli-
cation of diabetes. There are multiple patterns of sensory
neuropathy, including sensory motor neuropathies and small-fibre
neuropathies.
Between 10 and 25% (approximately 16%)
4
of diabetic patients
may experience painful neuropathy. In 10–20% of patients, the
symptoms are severe enough to warrant treatment. It is frequently
unreported in 12.5% of patients and untreated in 39% of patients.
5
Pain may be mild and intermittent or severe and unremitting,
resulting in diminished quality of life. Patients describe the pain as
burning, scalding, lancinating, tingling or having electric shocks.
Most frequently, the symptoms are restricted to the feet, but any
nerve may be affected, including the legs, arms, hands and fingers.
Patients may also experience allodynia or hypersensitivity of the
skin.
Symptoms may become chronic and worsen over time, but in
some patients, improvement and occasionally resolution occurs
over a period of years. A decrease in pain may imply either a
gradual recovery of nerve function or a worsening of the condition,
with progressive nerve death.
Painful neuropathy is also associated with and complicated
by sleep and mood disorders and these tend to aggravate the
symptoms considerably. Depression unrelated to the pain itself and
other causes of pain must be excluded.
There are significant social, psychological and financial stresses
imposed on patients with chronic painful neuropathy. The financial
burden is made even more acute by painful neuropathy being
excluded from the list of prescribed minimum benefit (PMB)
conditions guaranteed for automatic full reimbursement by medical
aid societies, the cost not automatically being covered as a ‘chronic
condition’.
Acute painful neuropathy may also be induced by rapid
correction of serum glucose, and this is called treatment-induced
diabetic neuropathy. In a report from Daby
et al.
,
6
acute painful
neuropathy developed two to four weeks after starting insulin in six
patients, four of whom were long-standing diabetics and in whom
the blood sugar was lowered from 15–33 mmol/l to 3.5–9 mmol/l.
Symptoms gradually improved over time (three to eight months).
Gibbons and Freeman
7
found that rapid glucose control
occasionally brought on acute nerve pain. Rapid weight loss also
did the same occasionally with no apparent cause. Improvement
occurred after 18 months of glucose control. The authors of this
article found that there was greater improvement in patients with
type 1 diabetes than in those with type 2 diabetes.
7
Pathophysiology of PDPN
The pathophysiological mechanisms underlying PDPN are complex and
beyond the scope of this article other than a brief outline (Fig. 4).
The peripheral nerve is made up of different nerve types. Within
the nerve, the small unmyelinated C fibres cluster towards the mid
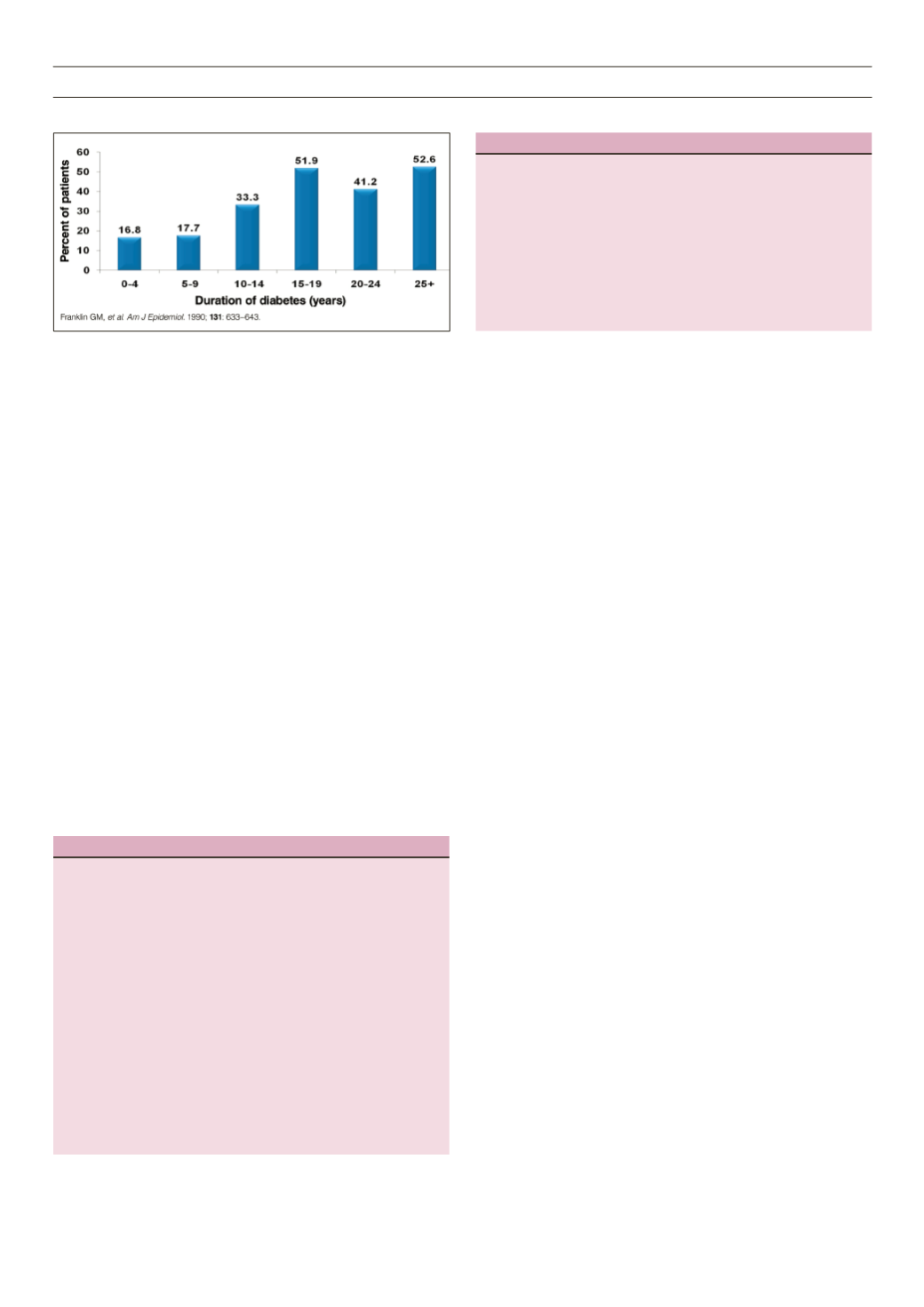
Figure 3.
Prevalence of DPN by duration of type 2 diabetes.
Table 1.
Differential diagnosis.
The differential diagnosis of neuropathy includes:
• Diabetes (painful neuropathy)
• Claudication
• Morton’s neuroma
• Osteoarthritis
• Radiculopathy
• Non-diabetic or inflammatory neuropathies
• Charcot’s neuropathy
• Plantar fasciitis
• Tarsal tunnel syndrome
• Fibromyalgia
• Connective tissue diseases
• Sarcoidosis
• Vitamin B
1
deficiency
• Paraproteinaemia
• HIV infection, neurotoxic drug exposure
• Paraneoplastic syndrome
• Coeliac disease
Table 2.
Risk factors for developing diabetic neuropathy.
Long duration of diabetes
Poor blood glucose control
Poor weight control
Male gender
Age above 40 years
High cholesterol level
High blood pressure
Alcohol usage
Cigarette smoking
Tall stature
Insulin use


