References: 1. The ACCORD Study Group. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus.
N Engl J Med.
2010;362;1563-1574. 2. Elam MB, Lovato LC, Byington RP, Bonds D, Leiter L, Crouse JR
et al.
Hypertriglyceridemia and Low HDL-C Predicts Fenofibrate Response in the ACCORD-Lipid Trial.Abstract 19724 AHA 2010
LIPANTHYL
®
200 mg. Each capsule contains 200 mg fenofibrate (micronised). Reg. No. 30/7.5/0494.
For full prescribing information refer to the package insert approved by the Medicines Regulatory Authority. Date of Publication of this Promotional Material: September 2012.Abbott Laboratories S.A. (Pty) Limited,Abbott Place, 219
Golf Club Terrace, Constantia Kloof, 1709 Tel No 011 858 2000. Promo. No: 0001-0912-K435-A-2218.
S3
Do statins go far enough in the lipid
management of your patients?
18
16
14
12
10
8
6
4
2
0
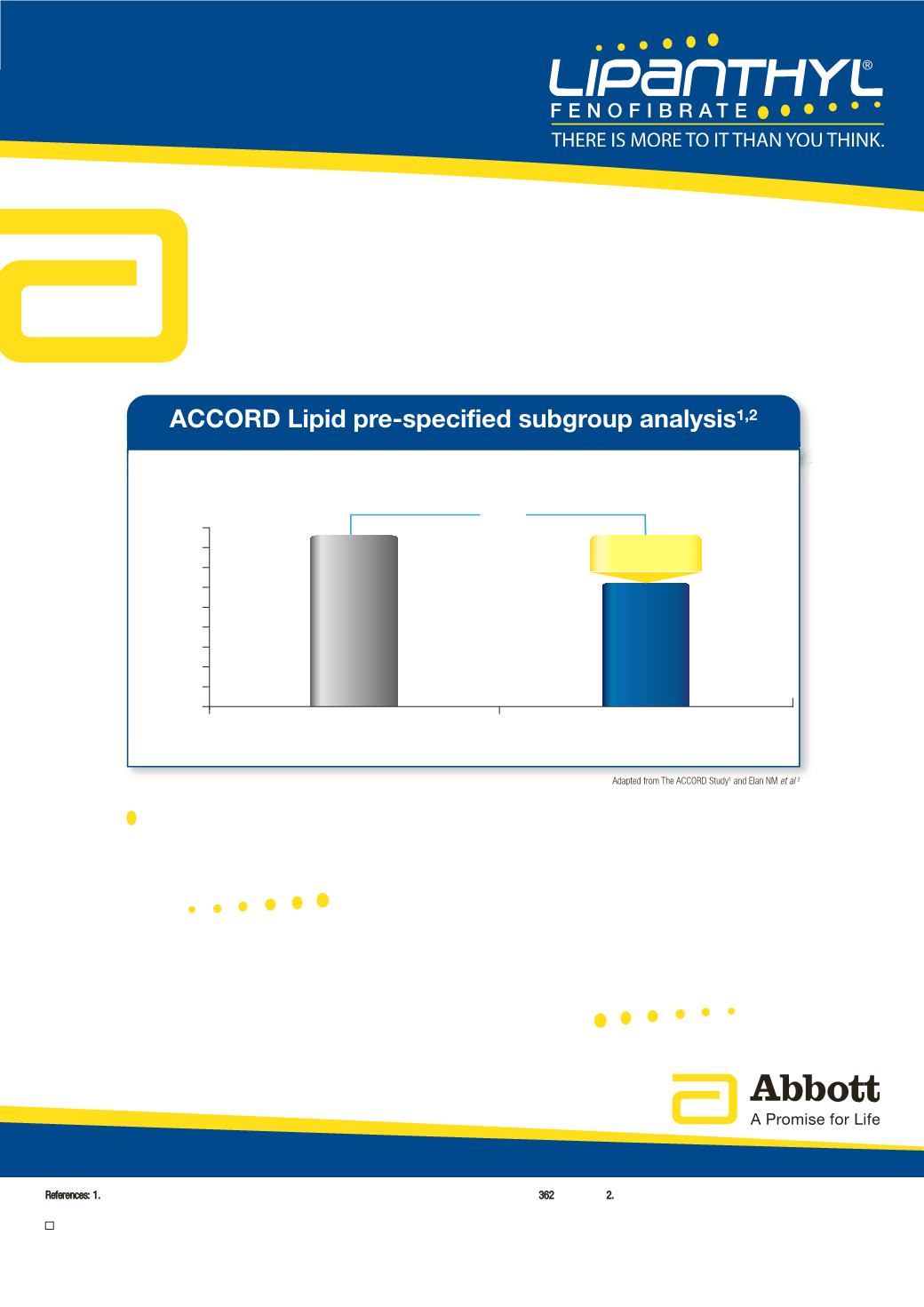
Proportion with
major CV event (%)
p=0.03
-31%
Simvastatin
(n=456)
LIPANTHYL
®
+ simvastatin
(n=485)
12.4%
Relative Risk
Patients with type 2 diabetes with TG
≥
2.30 mmol/l and HDL-C
≤
0.88 mmol/l
17.3%
GO BEYOND the STATIN QUO
Aim for Broader Lipid Management
Effective in reducing residual CV risk associated with elevated triglycerides
and low HDL-C in your statin-treated patients with type 2 diabetes
1


