SA JOURNAL OF DIABETES & VASCULAR DISEASE
REVIEW
VOLUME 8 NUMBER 1 • MARCH 2011
11
involves an attempt by one or more appropriately trained persons
to help the individual or family to:
• comprehend the medical facts, including the diagnosis,
probable course of the disorder, and the available management
• appreciate the way heredity contributes to the disorder and the
risk of recurrence in specified relatives
• understand the alternatives for dealing with the risk of
recurrence
• choose a course of action which seems to them appropriate
in view of their risk, their family goals and their ethical and
religious standards, and to act in accordance with that decision
• make the best possible adjustment to the disorder in the
affected family member and/or to the risk of recurrence of that
disorder.
In 2006 the National Society of Genetic Counselors adopted a
slightly different definition. This stated that genetic counselling is
the process of helping people understand and adapt to the medical,
psychological and familial implications of genetic contributions to
disease. This process integrates the following:
• interpretation of family and medical histories to assess the
chance of disease occurrence or recurrence
• education about inheritance, testing, management, prevention,
resources and research
• counselling to promote informed choices and adaptation to the
risk or condition.
14
Establishing and interpreting the family history
Vital to the process of counselling individuals (or for children and
their parents) with or at risk of T1D is the documentation of a
family history, including relevant clinical features of those reported
to be affected. The family history must be at least three generations
and should be depicted as a family tree or genogram to facilitate
interpretation of the data.
Important information that can be obtained from the family
history begins with the biological relationship between affected
members, and the presence and degree of consanguinity if present.
For those reported as affected, it is necessary to determine the
regions where they were raised and live, the type of their diabetes,
their age at onset and progression of the disease, as well as the
presence of co-morbidities such as deafness, optic atrophy or
skeletal dysplasia. From this information, the clinician should be
able to identify monogenic forms of diabetes such as MODY,
neonatal diabetes, Wolfram syndrome, mitochondrial (part of type
1B) and X-linked forms.
Many cases of type 1B diabetes (insulin-dependent diabetes
without evidence of antibodies) are reported to occur in persons of
African or Asian descent.
4
It is therefore important to consider other
forms of diabetes, as shown in Table 1, in South Africa. Craig
et al
.
tabulated the differences between T1D and monogenic forms.
4
Genetic syndromes that are sometimes complicated by diabetes,
such as cystic fibrosis (CF), Bardet-Biedl (BBS), Prader-Willi (PWS) and
Turner syndromes may also be suspected or identified.
10-12
Where
such syndromes are suspected and/or identified, the individual
and family need to receive specific care, including counselling,
from a medical geneticist or genetic counsellor if available. Such
counselling is outside the brief of this article.
Despite a positive family history being obtained in about 10%
of patients with T1D, a clear inheritance pattern for it has not been
identified. T1D is considered to be multi-factorial in inheritance with
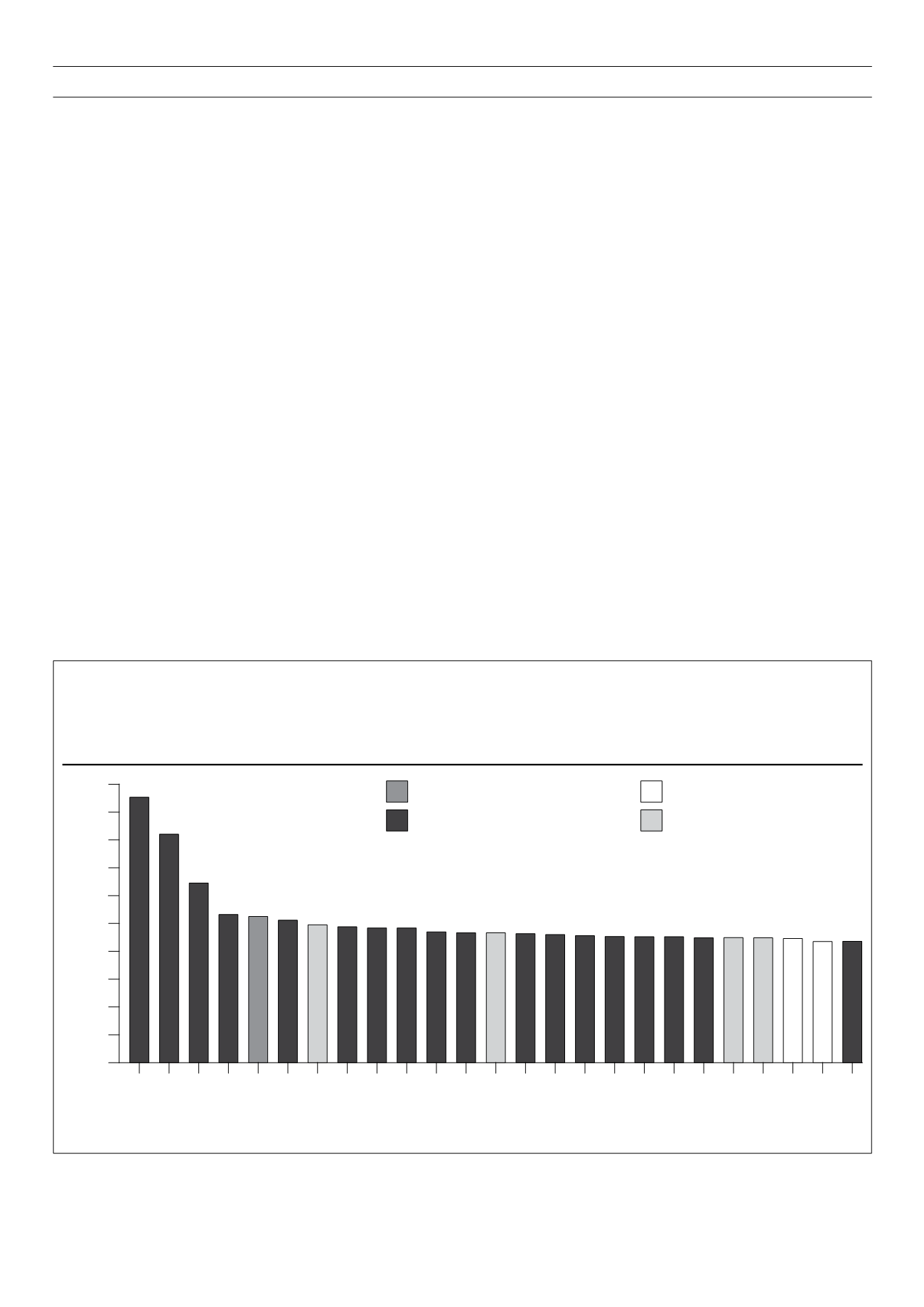
Figure 1.
Putative functions of non-HLA associated loci in T1D. The
y
-axis indicates the best estimate of the odds ratio for risk alleles of each of the indicated
loci, based on current publications. The HLA region’s odds ratio is not indicated but is approximately 6.8. On the
x
-axis, possible candidate genes
within the genomic regions with strong associations are indicated. The bars are coloured in accordance with known functions of these genes and to
suggest a probable role in the susceptibility to T1D. At
IL2RA
and
TNFAIP3
there is evidence of two different effects on risk and they therefore appear
twice. From Concannon
et al.
2009, reproduced with permission.
Odds Ratio
Locus
Insulin production and metabolism
Immunity
Protection from beta-cell apoptosis
Unknown function
2.50
2.25
2.00
1.75
1.50
1.25
1.00
0.75
0.50
0.25
0.00
INS
PTPN22
IL2RA
SH2B3
ERBB3
PTPN2
CLEC16A
CTLA4
IL18RAP
PTPN2
CCR5
IFIH1
CTSH
CD226
IL2RA
PRKCQ
IL2
CACH2
UBASH31
RGS1
IL7RA
CIQTNF6
TNFAIP3
TRNFAIP3
TAGAP


