
RESEARCH ARTICLE
SA JOURNAL OF DIABETES & VASCULAR DISEASE
54
VOLUME 17 NUMBER 2 • NOVEMBER 2020
dysfunction is known to precede systolic impairment in type 1
diabetic patients
18
and in STZ-induced diabetic rats,
19,20
and that
diastolic dysfunction is a common cause of systolic heart failure in
diabetes,
23
the improvement of systolic activity by Mg
2+
observed in
our study could be secondary to the diastolic modulation observed
in the acute diabetes disease model.
14
In the present study, there were no detectable cardiac
morphological changes to account for the contractile dysfunction
induced by diabetes. The gross heart weight was unaltered, and
histologically, there was neither a change in cardiomyocyte size nor
interstitial fibrosis. In addition, there was no significant coronary
perivascular fibrosis or cellular infiltrates that would have been
expected to impair coronary perfusion, a finding that was also
consistent with the lack of change in coronary flow rate observed
in this study.
These findings are in agreement with those in other studies on
chronic STZ-induced diabetic rats in which the cardiac dysfunction
was not accompanied by histological evidence of cardiac cellular
hypertrophy or fibrosis.
20
In contrast, other studies in chronic STZ-
induced diabetic rats showed that there was cardiac dysfunction
together with histological evidence of cardiomyocyte hypertrophy
and fibrosis.
24
These histological differences are likely to be related
to the duration of diabetes, given that in diabetic patients, the
deposition of collagen in cardiac tissue only becomes more
prominent in the later stages of heart failure when there is a low
ejection fraction.
25
In our study, there were no significant cardiac histological
changes to account for the effect of Mg
2+
. Taken together, the lack
of histological alterations in our study supports the concept that
the nature of diabetic ventricular dysfunction and the effect of Mg
2+
were functional, rather than structural.
The STZ-induced decrease in heart rate observed in the present
study and its prevention by Mg
2+
were consistent with our previous
findings in the acute-diabetes model where the relative bradycardia
was also observed
in vivo.
14
The bradycardia in STZ-induced
diabetic rats has also been reported in other studies,
20,26
and has
been attributed to cardiac autonomic synaptic degradation,
26
but
the basis of the bradycardia in our study remains unclear. In this
study, the bradycardia seemed to be unrelated to the modulation
of cardiac electrical activity since there were no significant changes
in ECG waves. The prolongation of the QT interval in diabetes was
probably related to changes in heart rate because the QT interval,
corrected for the heart rate (QTc), was not significantly different
among the treatment groups. Taken together, the occurrence of
bradycardia both
in vivo
and
ex vivo
and its prevention by Mg
2+
suggest that these effects were intrinsic to the heart.
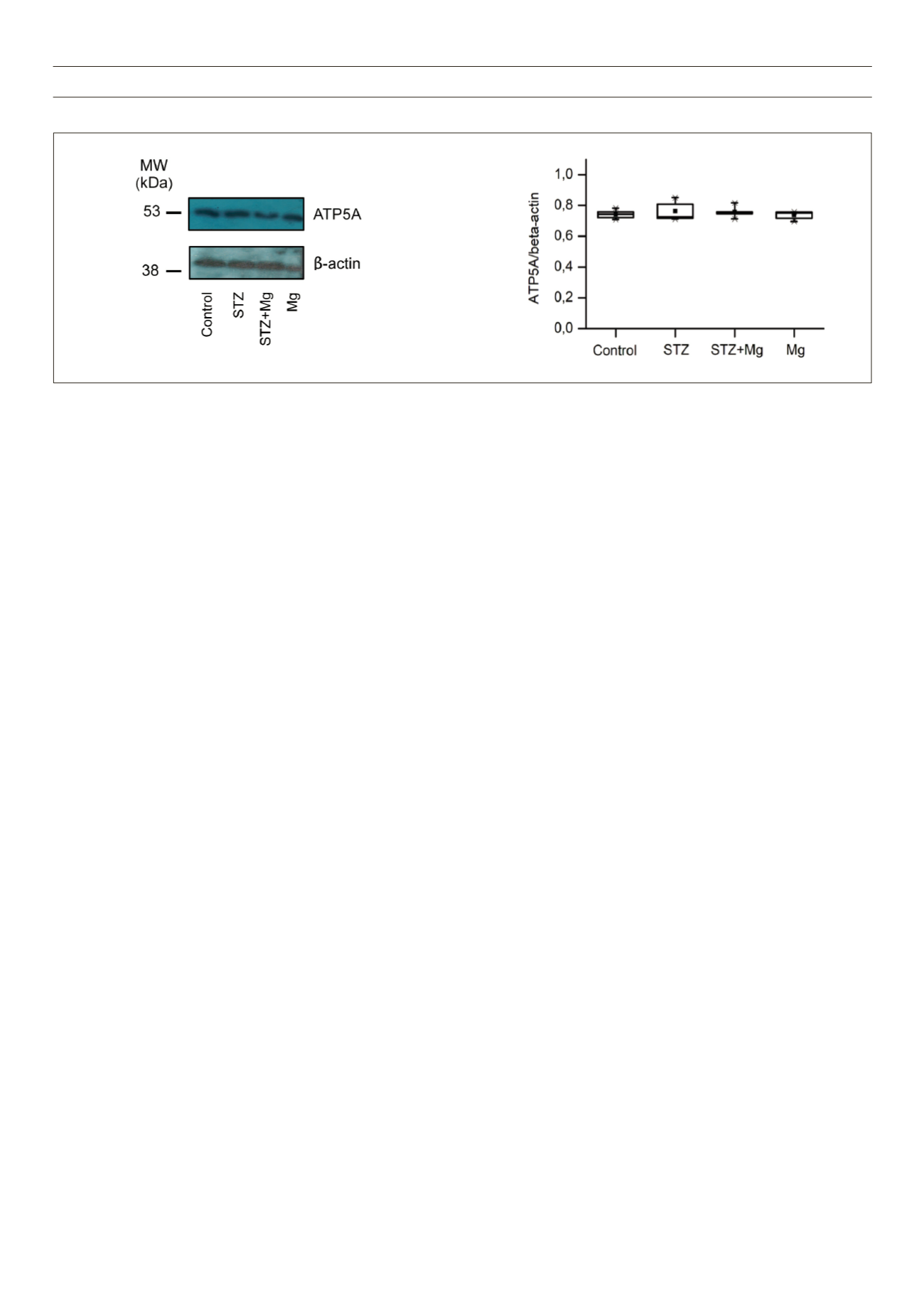
Despite the improvements in cardiac function by Mg
2+
, there
were no significant differences in the cardiac expression of ATP5A,
a cardiac biomarker that could have accounted for the Mg
2+
effects
at a molecular level. Mg
2+
is a key co-factor of several co-enzymes
that may alter the cardiac metabolic status, it also contributes to
cellular energetics via its coupling with ATP to form MgATP,
13
and it
may therefore alter mitochondrial function. However, in our study,
there were no changes in the metabolic indices, as was indicated by
the mitochondrial metabolic component ATP5A. Therefore, further
molecular studies such as those evaluating aspects of mitochondrial
fusion/ fission are required to elucidate the role of Mg
2+
at the
cardiac cellular level.
Limitations of this study include the use of an artificial, STZ-
induced diabetic model, in which the Mg
2+
effects may not be
readily translatable to the natural disease. However, the STZ-induced
diabetic rat model is known to mimic diabetic complications in
humans.
21
We also previously showed the value of this disease
model in that, apart frommimicking type 1 diabetes, it also exhibited
features of type 2 diabetes, such as dyslipidaemia.
14
Also, the clinical
relevance of the Mg
2+
dose used in this study remains unclear, given
that that the dose (270 mg/kg) is higher than that used via the
oral route in human supplementation, and is only comparable to
the loading intravenous/intramuscular dose used in eclampsia (~
230 mg/kg).
27
Nonetheless, the peak increases at 3.5 hours of ~
0.7 mmol/l, achievable under our experimental conditions,
15
are still
within the therapeutic ranges of other clinical conditions.
27
Finally,
since the experiments were performed at cardiac tissue level, the
presence of an intracellular Mg
2+
deficit cannot be excluded, and
therefore requires further investigations at a cellular level.
Conclusion
The results of this study show that Mg
2+
improved cardiac contractile
function and stabilised heart rate in the STZ-induced chronic
diabetes rat model, without preventing metabolic derangements
A
B
Fig. 5.
Western blot analysis of mitochondrial ATP5A protein. A: Representative Western blot film images of ATP5A and the corresponding
β
-actin in ventricular
tissue of different hearts. B: Summary data of the fold-expression of ATP5A, normalised to that of
β
-actin. Data are shown as box plots and the mean (
n
);
n
= 3 per
group.



















