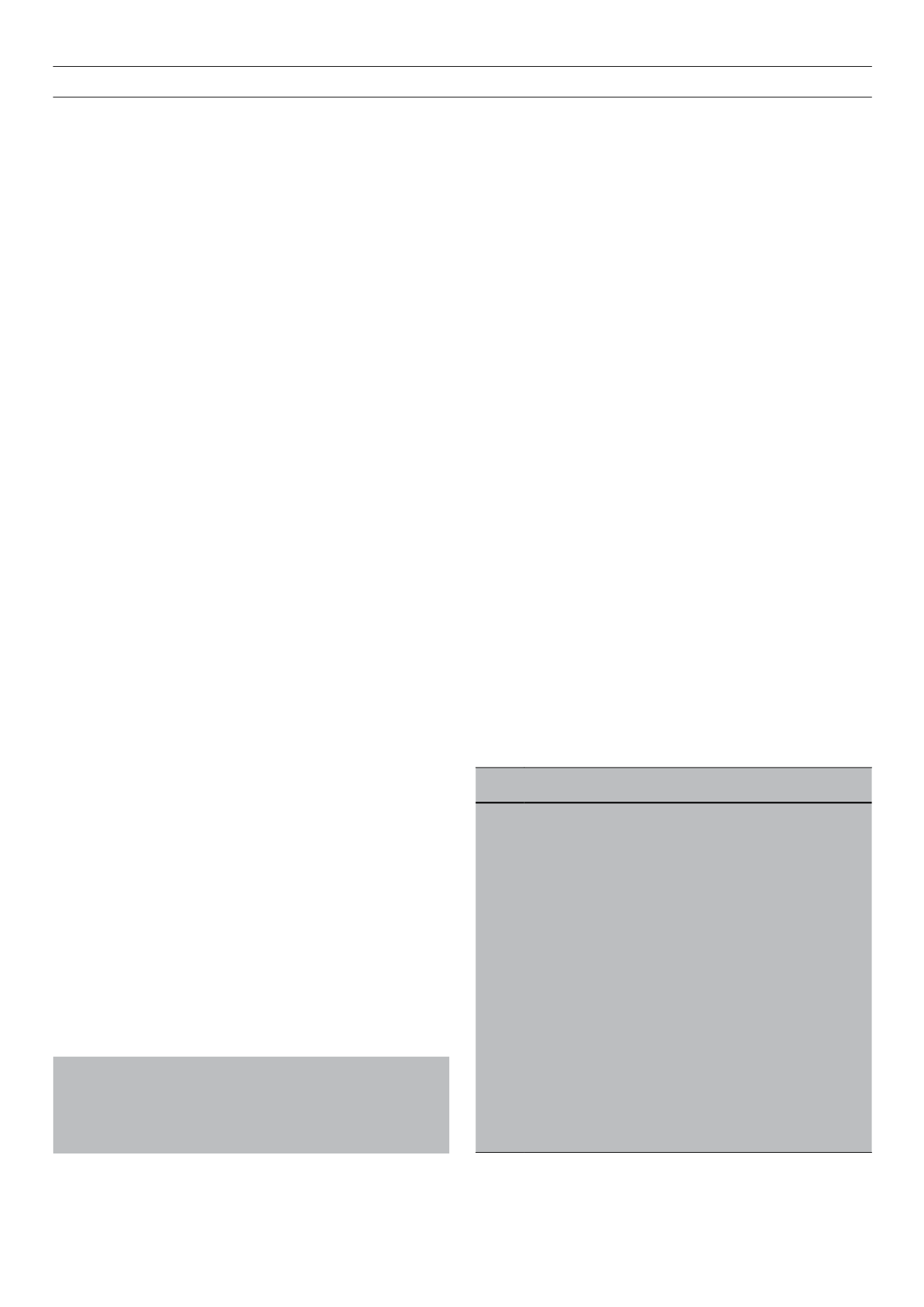
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
10
VOLUME 8 NUMBER 1 • MARCH 2011
Genetic counselling in type 1 diabetes mellitus
BERTRAM D HENDERSON
Introduction
I
n 1976, James Neel, the renowned human geneticist, titled a
book chapter ‘Diabetes mellitus: a geneticist’s nightmare’.
1
Eleven years later, in 1987, Harry Keen wrote ‘The genetics of
diabetes: from nightmare to headache’,
2
closing with the title of
Rotter’s 1981 article ‘no longer a nightmare but still a headache’.
3
Since then a wealth of information has been accumulated about
diabetes mellitus.
Diabetes is now considered a group of metabolic disorders
characterised by chronic hyperglycaemia resulting from defects in
insulin secretion, action or both.
4
In type 1 diabetes mellitus (T1D)
there is destruction of the pancreatic islet
β
-cells. T1D is divided
into type 1A where there is T-cell mediated destruction of the
pancreatic islet
β
-cells, and type 1B where the destruction of these
cells is not immune mediated.
4
The full classification of diabetes is
set out in Table 1.
4,5
Diabetes mellitus, including T1D, is being diagnosed more
frequently than previously,
4,5
thus putting a greater demand on
health resources. The identification of at-risk individuals in order to
embark on preventative strategies has therefore become a valuable
approach to the reduction of the diabetic burden of disease.
The genetics of type 1 diabetes mellitus
The advent of new, powerful, analytical genetic techniques
including candidate gene approaches, positional cloning, genome-
wide association studies and single nucleotide polymorphism (SNP)
profiling has resulted in a vast amount of genetic information
becoming available in a short period of time.
6,7
T1D has been
linked to more than 50 chromosome loci with at least 40 candidate
causal genes identified. The most consistent and strongest linkage
is with human lymphocyte antigen (HLA) on chromosome 6p21.3
and specifically the DRB1, DQA1 and DQB1 (DR3/DR4) loci, which
accounts for 40% of familial aggregation.
4,5,7,8
There is also strong linkage to genes involved in T-cell function
(
PTPN22, CTLA-4
), the insulin gene (
INS
), interleukin-2 (
IL2RA
) and
an interferon-induced helicase gene (
IFIH1
).
5,7,8
Some of the other
loci have inconsistent results and a number are linked with up
to five other autoimmune disorders.
7,8
Readers can access www.
t1dbase.org for more detail on the genes within these loci.
9
This
plethora of information makes the understanding of the genetics
less complicated than before but still poses a number of challenges,
particularly for the healthcare practitioners with limited genetics
training and knowledge.
Differential diagnosis of insulin-dependent diabetes
in children
The majority of children presenting with insulin-dependent diabetes
mellitus (IDDM) will have type 1A diabetes. A large United Kingdom
study found that only 0.7% of children with IDDM had one of the
rare non-immune mediated forms. Their causes include defects
in insulin secretion, the so-called MODY (maturity-onset diabetes
of the young) where seven genes are known to be involved and
insulin receptor mutations result.
Mutations in the glucokinase gene cause a non-progressive
hyperglycaemia. Diabetes in infants under six months of age is
usually due to mutations in the
KCLN11
gene. Other disorders
to consider, in which diabetes is part of the clinical features, are
the autosomal recessive Alstrom, Bardet-Biedl, Wolfram (or DID-
MOAD), Wolcott-Rallison or Rogers syndromes.
Mitochondrial (same mutations that cause MELAS) mutations
can also present with diabetes in childhood. Monogenic forms of
diabetes are to be considered when diabetes is diagnosed before
six months of age, there is an autosomal-dominant family history
of diabetes, consanguinity, mild fasting hyperglycaemia in young
people and extra-pancreatic features such as deafness, optic
atrophy, liver disease or epiphyseal dysplasia.
10-12
Genetic counselling
The American Society for Human Genetics in 1975 adopted the
description of genetic counselling
13
as a communication process that
deals with the human problems associated with the occurrence, or
risk of recurrence, of a genetic disorder in a family. The process
Correspondence to: Bertram D Henderson
Division Human Genetics, University of the Free State, Bloemfontein
Tel: +27 (0) 51 405-3046
e-mail:
S Afr J Diabetes Vasc Dis
2011;
8
: 10–13.
Table 1.
The classification of diabetes mellitus
Type 1
β
-cell destruction, usually leading to absolute insulin deficiency
1A Immune mediated
1B Idiopathic (non-immune)
Type 2 Non-insulin dependent diabetes mellitus. May range from
predominantly insulin resistance with relative insulin deficiency
to predominantly secretory defect with or without insulin resistance
Type 3 Other specific types
3A Genetic defects of
β
-cell function
3B Genetic defects in insulin function
3C Diseases of exocrine pancreas
3D Endocrinopathies
3E Drug or chemically induced
3F
Infections
3G Uncommon forms of immune-mediated diabetes
3H Other genetic syndrome sometimes associated with diabetes
Type 4 Gestational diabetes